Plant diversity, plant species and populations
Author: Mária Höhn
5. 1. Biological diversity on Earth
A huge variety of life forms exist on Earth. They are linked to different terrestrial, marine and freshwater habitat types. This variability can be described by the term: biological diversity.
The diversity of life evolved gradually during Earth’s history and it has changed much over the geological times. Some geological ages are characterized by massive species extinctions, whereas other periods whitnessed important evolutionary and mass dispersion events. Changes in species diversity depend on geo-historical events, climate of geological ages, paleogeographical and geomorphological factors and the genetics and ability of organisms to adapt to changing conditions.
A term closely related to biological diversity (biodiversity) is geodiversity, which represents the diversity of all environmental components within an area.
If we ask a simple question – How many species live on Earth? -, it is hard to give a clear answer. First we have to answer another question: Do we know all species that live on Earth?
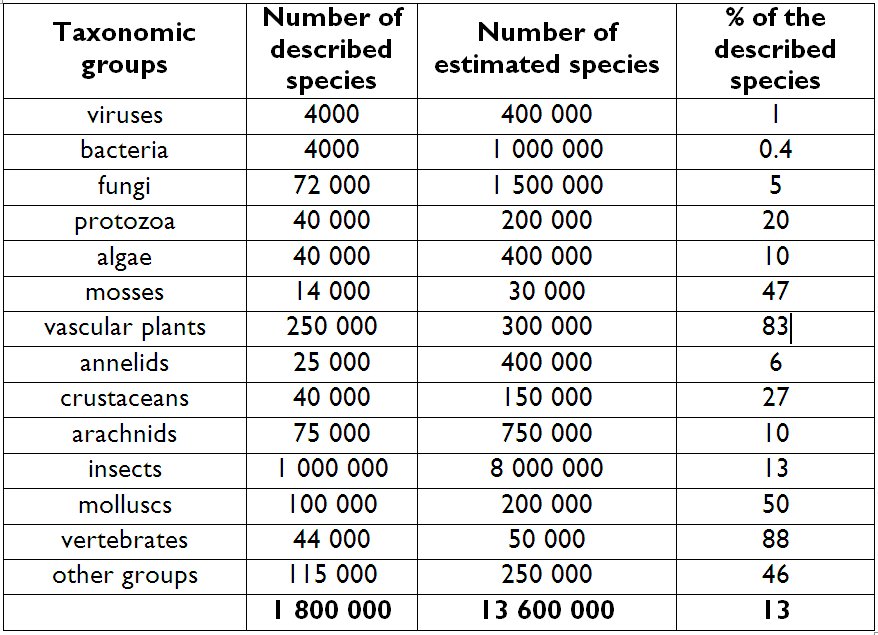
The answer clearly is: no. Although the discovery and description of species (our learning process about species) started a long time ago and several plant and animal species have already been described in the antiquity, we still only have estimates about the number of species that live on Earth today. The Table I. shows that there are significant differences between the known and estimated numbers of species in different taxonomical groups.
1. Table: Known and estimated number of species on Earth (Cristea and Denaeyer 2004)
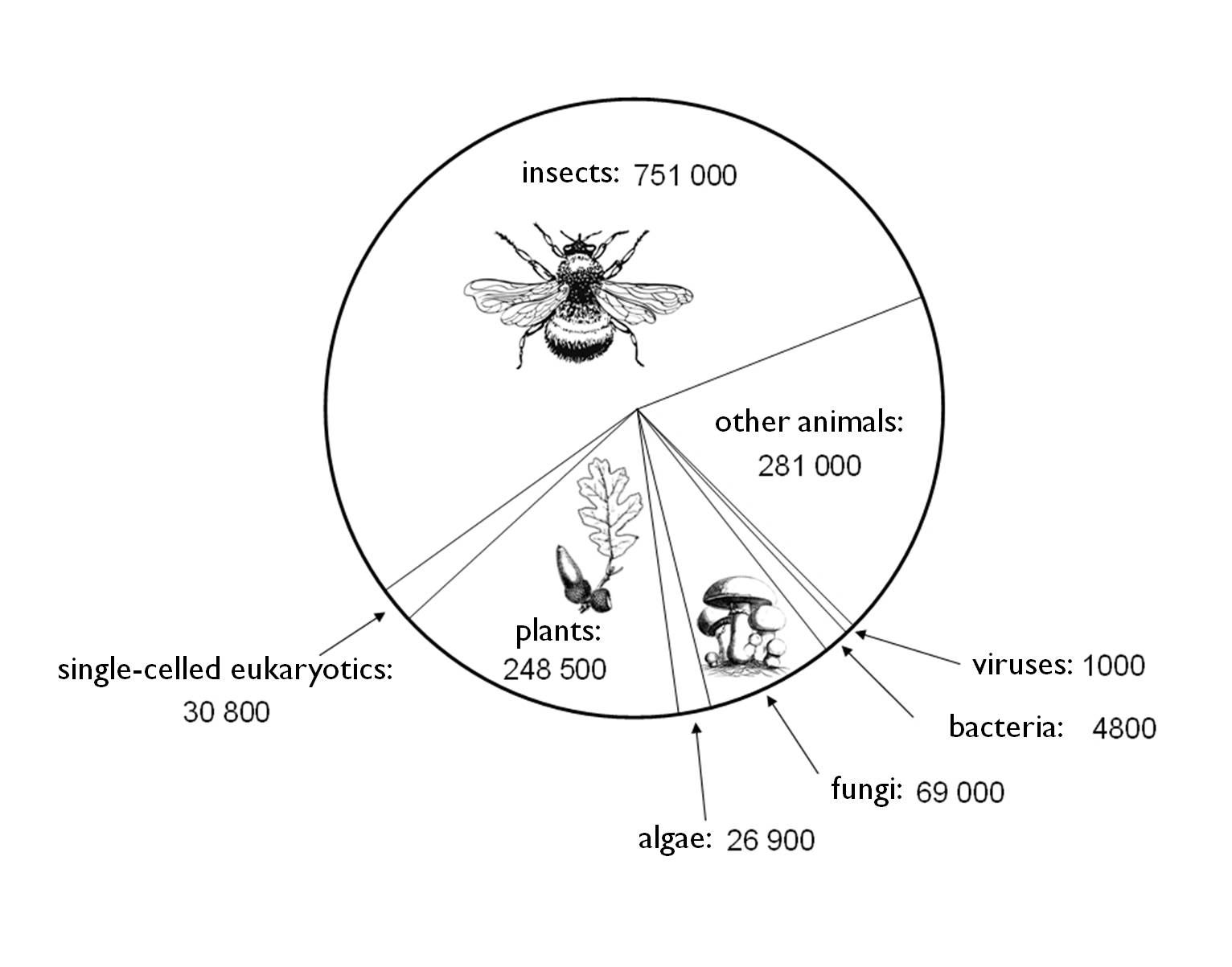
1. Figure: The partitioning of species diversity among different taxonomic units (Standovár and Primack 2001)
The best known groups are the higher plants and animals. From among the insects only 13% of the estimated 8 million species are currently known to the scientific community. According to the above-mentioned authors and considering the estimated number of 13 million species that exist on Earth we can say that the 1.8-2 million species recorded so far represent a mere 13% of the total species that might exist on Earth.
The partitioning of the known species among different taxonomical units is shown in Figure 1.
Other sources (Mora et al. 2011) place the current estimated species number to approximately 8.7 millions (± 1.3 millions), of which about 2.2 million species live in marine habitats. For details, see the following website: http://www.diversitas-international.org/activities/research/biogenesis.
Regardless of how we calculate these values, it is evident that on the scale of approximately ten million species, higher plants (which are believed to be well known) with 280-300 thousand recorded species represent a small portion of Earth’ diversity.
The higher plants (Gymnosperms and Angiosperms) which disperse by seeds were the most successful in colonizing the terrestrial habitats. Seeds as reproductive organs provided the opportunity for the embryo within the seed to survive during adverse environmental conditions. Germination, the development of a new life, starts only if the external environmental conditions are suitable (for details see Chapter 4). The functional structures of the seed support the embryo at the beginning of the germination.
How long can a species exist it is also hard to answer. Very different sets of species have lived during different geological ages, and Earth’s diversity has changed permanently. Since Darwin (who introduced the concept of evolution) we know that changes in species diversity at geological scales are driven by evolutionary forces.
Based on a synthesis of recent phylogeografical and paleobiological research results, the Hungarian scientist Attila Borhidi (2009) delimited four important milestones in the evolution of plant species. These are basically developmental phases in which the initial periods are characterized by a relatively rapid and massive evolution of new structural types and species:
1.) The evolution of the prehistoric marine life (Palaeozoic Era – Cambrian Period). Almost all structural-functional life forms suitable for the marine environment were developed (9 phylums of algae and with the exception of vertebrates, 17 phylums of animals)
2.) The conquest of the land by plants (Devonian Period, the oxygen content of Earth's atmosphere increased to 10%). The UV radiation on Earth’s surface decreased considerably due to the formation of the ozone layer.
A more efficient water transport system, the vascular tissues developed→ evolution of vascular plants (Tracheophytes with specialized lignified tissues able to transport water and nutrients).
3.) Gymnosperm species (end of Devonian and beginning of Carboniferous Period). The evolution of seed plants.
4.) The explosive spread of Angiosperms (starting the end of the Permian Period: the mainland bursts into bloom).
A huge diversity of fruits and pollination types and fruit dispersal types evolve. Meanwhile, a diversification of animal species takes place, because:
‘ The animals – including humans – live their lives on Earth as plants‘ guests on the Planet .’
Different diversity indices are being used to quantify the biological diversity of an area or of a habitat.
The most commonly used indices are the α, β and γ-diversity:
α – the number of species in a given locality (the number of taxa),
β – taxon diversity of a series of habitats, a ratio, a similarity index,
γ – the diversity of a larger region, e.g. of an entire ecosystem or the diversity of a landscape, or of a biome.
The beta diversity allows us to compare several areas of a larger region along a transect. The simplest way to calculate it is:
β1 – γ/ α1
β2 – γ/ α2
Diversity can be also characterized by indices calculated from the relative frequency of species or their individuals in a community:
A simple diversity index is the Shannon-Wiener index (H)
- it is one of the most commonly used diversity indices „borrowed” from the information theory:
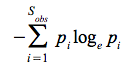
where : pi – is the relative frequency of the „ ith” species in the community;
Another commonly used diversity index is the Simpson index:
- it calculates the probability of an individual to belong to the same species as the specimen recorded previously.
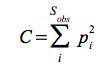
where 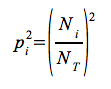 and the final index is:
and the final index is: 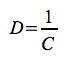
Ni – the number of individuals that belong to species i
NT – number of individuals in a sample
The Jaccard or the Soerensen indices allow us to compare the diversity of different habitats, areas.
In addition to the species number (richness), the indices of species diversity take into account the frequency, distribution and evenness of each species. For example, a habitat that has higher species evenness (i.e., the probability of occurrence of difference species is similar) is more diverse than a habitat of low species evenness.
In addition to the diversity indices, the biological diversity can be evaluated at different other levels:
-
- individual level:
- morphological diversity
- genetic diversity: calculated from allele frequencies (α diversity)
-
- species level:
- the diversity of organisms and populations (see before)
-
- ecosystem level:
- species number / area: quantifiable by various indices
- phylogenetic diversity, the range of different phylogenetic lineages and their relationship
- ecological diversity: the diversity of environmental charactistics
In a larger sense we can also talk about the diversity of the:
- life forms
- number of habitat types
- bedrock, soil, topography and hydrogeology
- landscape elements
Why is diversity important in nature? What are the benefits of a higher biological and geological diversity?
- several and more diverse habitats can support more organisms;
- higher habitat diversity results in higher biodiversity at different organizational levels;
- the more organisms in a habitat, the more complex relationships can develop between them;
- if the relationships between organisms are more or less constant, the systems works better;
- the systems become more stable due to the complexity of interactions.
- the systems develop self-sustaining capacity!
In conclusion:
Higher diversity provides increased stability and this is the way nature works!
The biological diversity has developed through the process of evolution. It is a confirmed fact, the greater the ecosystems’ diversity, the less energy it is required for its maintenance. The preservation of diversity is a successful survival strategy for most ecosystems.
The increase of diversity in any system is of course a definite process. Species-rich ecosystems may restrict the further diversity increase by self-regulating its internal relationships. This prevents the system from increasing its species numbers indefinitely, which could eventually lead to chaos (Figure 2).
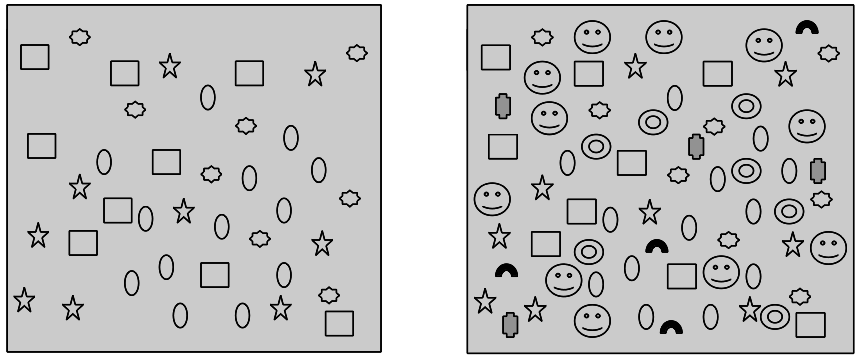
Figure 2: The distribution and density of individuals (icons) of different tree species in a forest habitat (after Standovár –Primack 2001). When individuals of a species are spatially too far from each other because of the high density of other organisms, that can constitute a reproductive barrier. This prevents the increase of the number of individuals through ecosystem self regulation.
Similar rules govern the functioning of anthropogenic ecosystems, and therefore cultivation practices should take into account the benefits of biological diversity. However, in modern agricultural ecosystems the quantity (biomass) and the quality (biodiversity) of the living matter is magnitudes lower than that of the traditional natural ecosystems (Vida 1996). The globalization and intensification of agricultural practices, the decrease of the number of cultivated varieties and the prevalence of only a handful of crop species and varieties led to instability. The worlds’ economy is vulnerable with only scarce gene stocks left and severely limited possibilities of breeding new and more resistant agricultural varieties. Today the conservation of agro-biodiversity has become a necessity, and in fact it is critical for the human well-being and security. These are very strong arguments to protect the gene pools of the agricultural and horticultural reserves left.
5. 2. Migration and dispersion in the plant world
The lifespan of natural species can differ significantly. During the geological history of the Earth, many species went extinct and in the same time new species evolved. Extinction is a natural process; however since humans begun dominating the ecosystems, the rate of species extinction has accelerated abnormally and it is significantly higher compared to the natural extinction rates. A wide variety of reasons drive species’ extinctions, but the excessive land use and habitat destruction are causing extinctions to happen too early.
During their life history species most often migrate, which involves major changes in their area of distribution. These changes take place on a long time scale, and can result in expanding or contracting geographic ranges or in shifts along a geographical gradient.
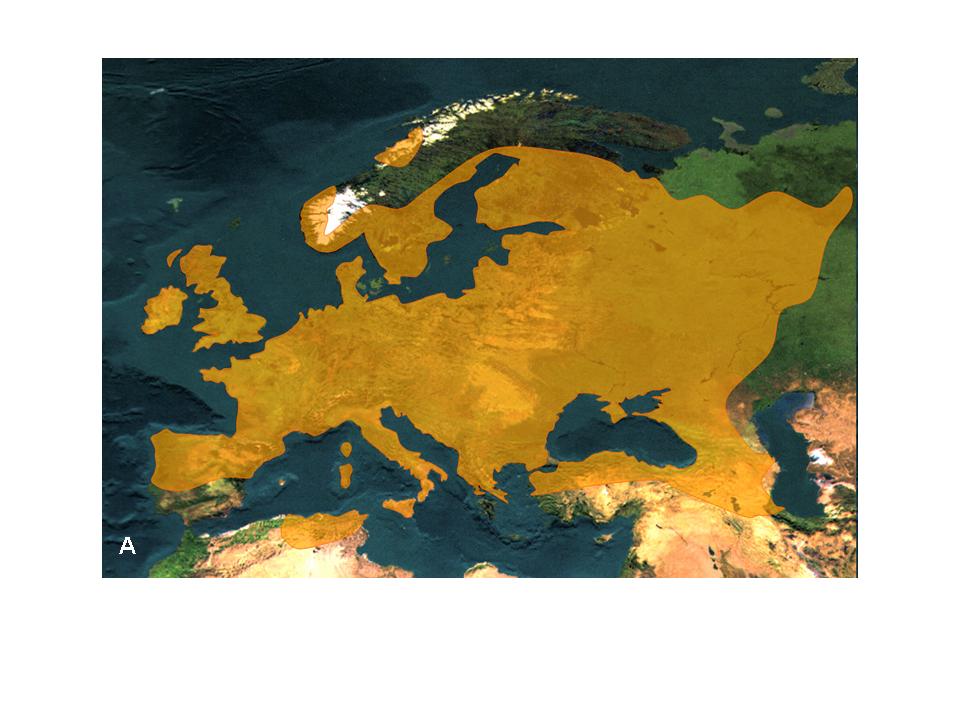
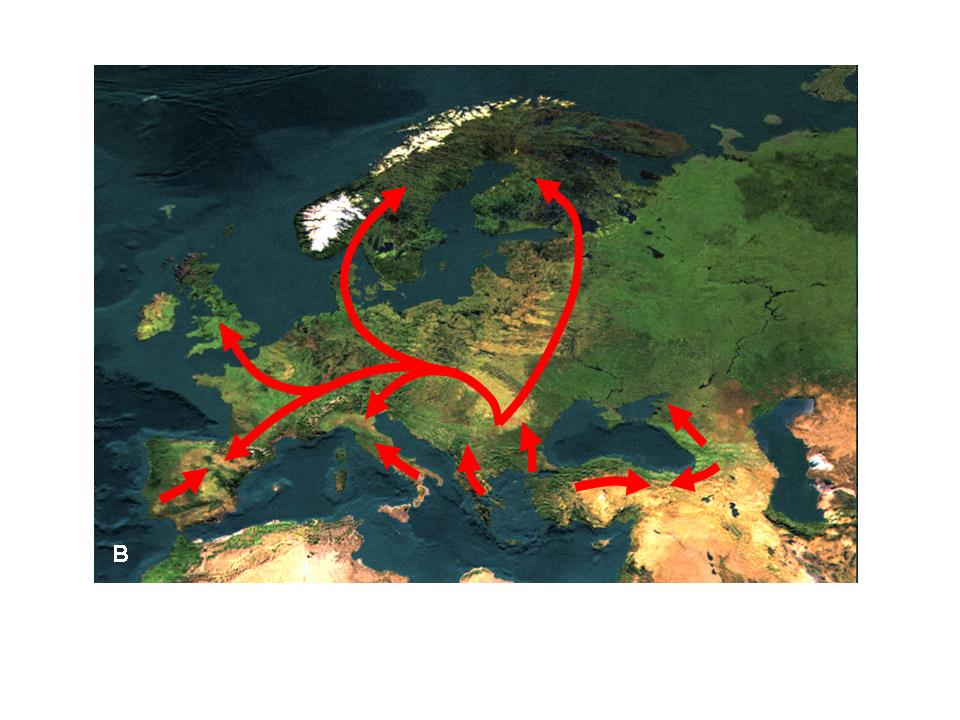
3. Figure: The distribution area of the black alder (Alnus glutinosa) (A) and its major postglacial migrations routes (B) (after Hewitt 2001)
Why do species migrate? One of the main migration forces is the extinction threat. Large-scale, global environmental changes can drive species to leave their area (e.g. the onset of the Ice Age); during this time species either become extinct or they colonize new territories (Figure 3). Through long-distance dispersal species can change their distribution area entirely, which means that the new geographic ranges are situated in a completely new geographic space (beyond-area migration). But usually the species “move” within their area of distribution, which can become fragmented and/ or rearranged over time. For example the Scots pine (Pinus sylvestris) extended northwards after the glacial period, colonizing territories freed from the ice sheet. It is now one of the most important forest-forming tree species of the Eurasian taiga (Figure 4). Meanwhile its former geographic range in Central Europe decreased during the postglacial warming period as the lowland habitats were being occupied by deciduous tree species. In the mountains Scots pine was replaced by spruce. However, after the climate warming Scots pine has not become extinct in South- and Central Europe. Because of its large ecological tolerance, populations were able to persist in some extreme habitats such as peat-bogs and steep rocky outcrops (Figure 5).
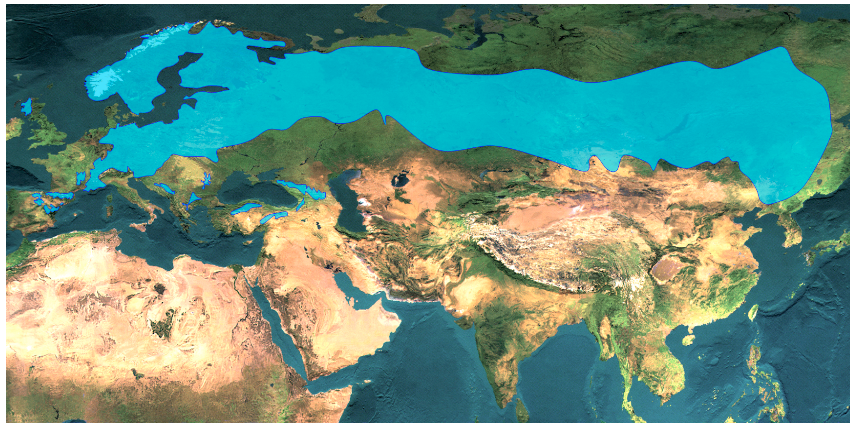
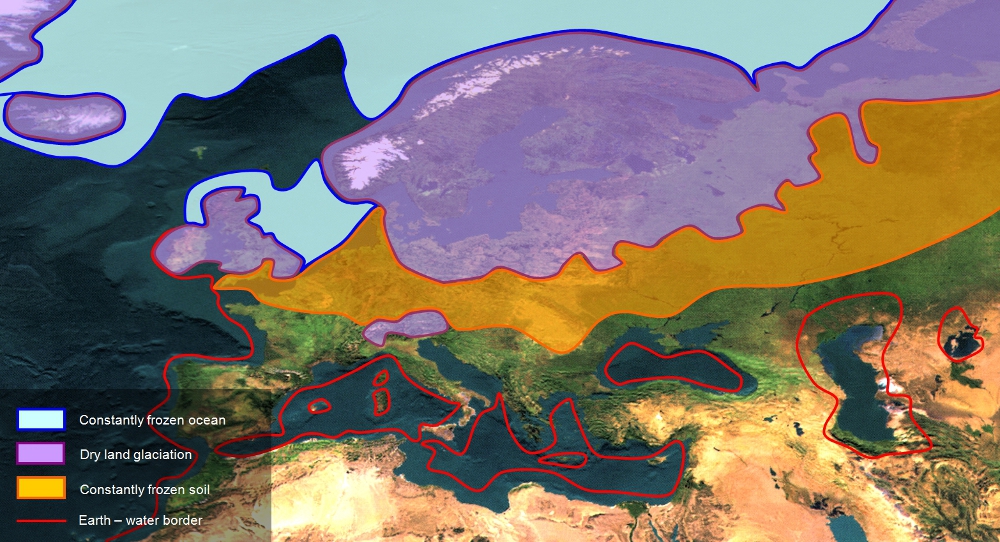
4. Figure: The current distribution of Scots pine (Pinus sylvestris) (from Euforgen database) (A). The extent of the ice sheet and the boundary of the permafrost during the Last Glacial Maximum, LGM (B) (Láng, Bedő and Csete 2003). The two maps above prove that most of Scots pines’ current distribution area was covered by ice during the Last Glacial Maximum.


Figure 5: Extreme habitats of Scots pine (Pinus sylvestris) in South-East Europe. A: on alcaline sandy subrate at Fenyőfő (Bakonyalja, Hungary); B: in the ‘Mohos’ peat bog near Tusnád (Ciumatu Mt. - Csomád., Romania); C: in the ’Poiana Stampei’ peat bog (Calimani - Kelemen Mt., Romania)
A small-scale shift in the geographic range can also mean survival for a species. For example, after the last ice age several cold-tolerant herbaceous species of the European middle mountains were forced to migrate to the adjacent higher elevation mountains, because their habitats were being colonized by thermophillous species. Meanwhile the melting snowcaps on the mountain tops uncovered new habitats which were colonized by these cold-tolerant species.
Dolomite has special physical and chemical characteristics, due to which the rock withering processes can produce a large variety of microhabitats. This is the reason why characteristic species of colder geological periods were able to survive in cold microhabitats on the Northern slopes of the middle mountains. Such species typically live in the high mountains of the Alps and of the Carpathian Mountains, e.g., Phyteuma orbiculare (Round-headed rampion) and Primula auricula (Mountain cowslip or bear's ear) (Figure 6).

6. Figure. Phyteuma orbiculare - Round-headed rampion (A) and Primula auricula – mountain cowslip (B)
The dolomite rocks of the Transdanubian Middle Mountains in Hungary preserved a large number of relict plant species. During the Ice Age, shorter warming periods alternated with longer cold periods. As a result, some species’ geographic range was severely fragmented and populations became isolated, yet they were able to survive within glacial refugia. Over long enough periods of time these isolated populations evolved into new subspecies or species. Such is the Linum dolomiticum, an endemic plant of the Pilis Mountain in Hungary, which survived only in a single locality at Kis-Szénás on Northerly dolomite slopes (Figure 7). It is considered an interesting and valuable endemic species of Hungary, whose history can be traced back to the Pleistocene times. Its closest relative is Linum elegans, native to sunny and rocky limestone outcrops of mountains in Greece, a long distance further South.

Figure 7. Linum dolomiticum at Kis-Szénás (Photo: László Udvardy)
5. 3. Population
Individuals of a species are only able to produce fertile offsprings when they meet each other, in other words when they share the same habitat and are capable of sexual reproduction. Therefore in a given habitat a species is represented by its population(s). Within their distribution area, species usually have several populations that occupy the ecologically suitable habitats. Importantly, a species consists of all of its living populations across its geographic range.
Definition of the population
The population is a group of individuals belonging to a species that live together in space and time. Due to interbreeding, there is a permanent gene flow (gene exchange) among individuals of a population.
A population is not simply a random gathering of individuals, but rather an organized group with specific structure and functions.
Dispersion of plant populations
The migration or “wandering” of plants is not (cannot be) the result of active locomotion. Because of the sessile lifestyle, plants can only disperse through propagules (e.g., seeds, spores, vegetative organs). The propagules are spread over large areas, and the ones that reach suitable conditions will germinate and may even establish a new population.
The dispersion is therefore a population-level event, whereas the migration of the species and changes in the area of distribution is the result of these population dynamic processes.
5. 3. 1. Characteristics of plant populations
Any plant or animal population can be described based on their general characteristics.
These are:
a) the number of individuals within the population
b) birth rate: the number of offsprings born within a time interval
c) mortality rate: the number of deaths within a time interval
Mortality can be characterized by two values: the biological mortality, which arises from the population-specific lifetime and the ecological mortality value, which depends on the environmental conditions. Under increased environmental stress (e.g. lack of nutrient or water supply, competition or antagonist populations), the ecological mortality rate of the population is higher.
d) age classes: the proportion of different generations within a population (Figure 8)
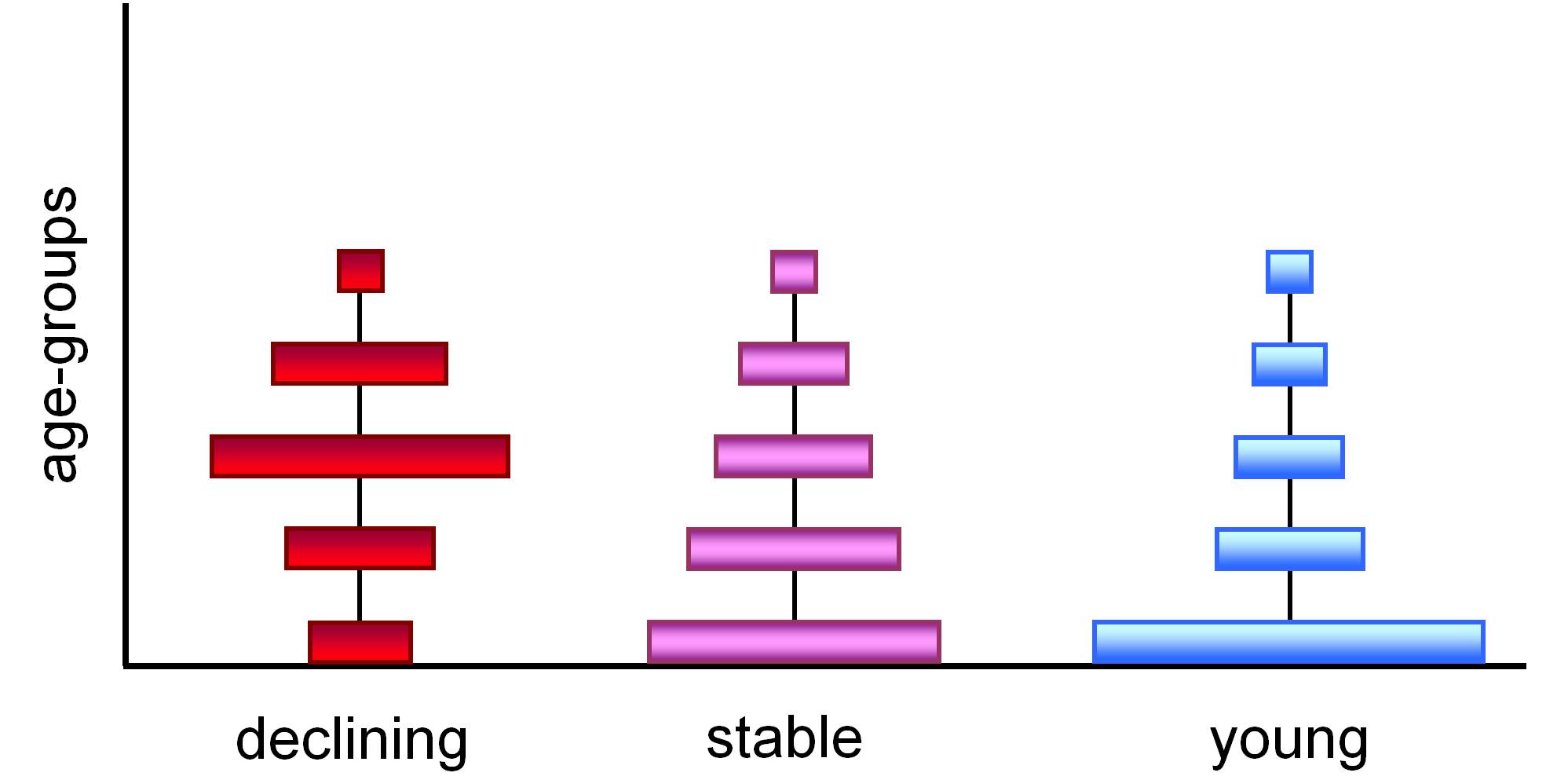
8. Figure. Aging (declining), stable and young populations.
e) population growth: Nt+1 = Nt + B - D +I - E
Nt – initial number of individuals at the beginning of the study interval; B- number of new recruits; D – number of deaths; I – number of immigrants; E – number of emigrants
f) Population density regulation
In order to suvive, populations must regulate their density (i.e., their number of individuals).
The production of offsprings is more or less continuous and a direct consequence of the reproduction ability of populations. This process can be described by the „r”-value, and is called population intrinsic rate of increase.
dN/ dt = rN , where rN is the rate of increase of the number of individuals within a time interval.
In an ideal environment (where all ecological needs are fulfilled), populations grow exponentially. This type of growth continues until environmental constraints start acting as limiting factors. When the number of individuals within a population reaches a critical number that corresponds to the carrying capacity (K) of the environment, the population growth will be regulated (the growth will slow down and eventually it will stop).
Populations can follow two main reproduction strategies: i) r-strategy and K-strategy(Figure 9).
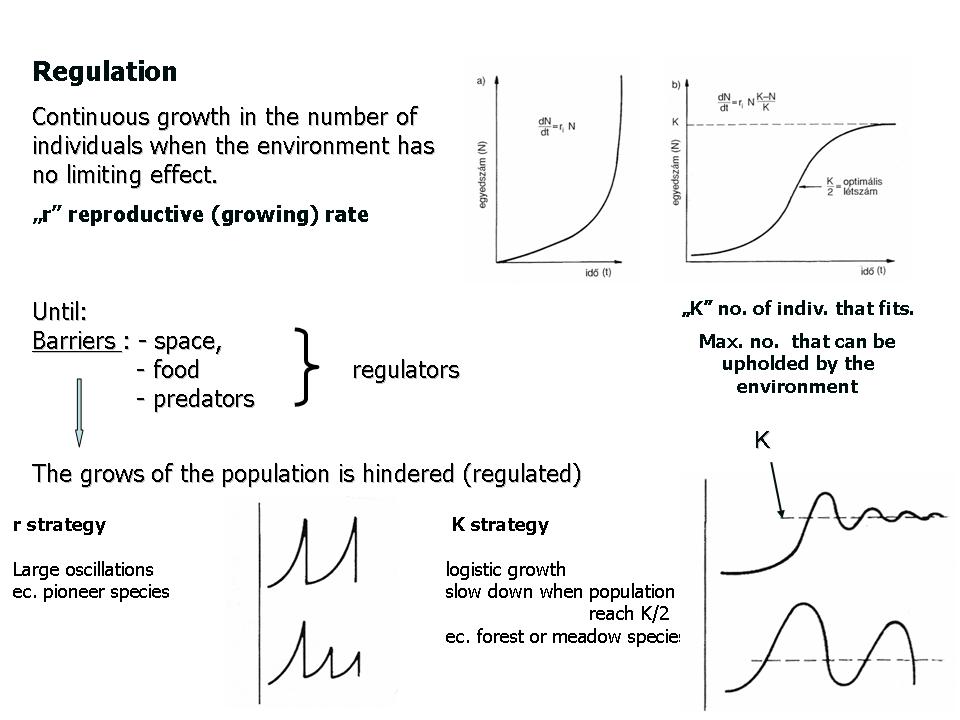
9. Figure. Population regulation, K- and r strategy
In species that follow the r-strategy the number of individuals within the population increases exponentially, but this is followed by mass mortality when the population runs out of resources. When conditions improve and a sufficient amount of resources becomes available, the population will increase rapidly and will follow again an exponential growth. This way the populations have a highly fluctuating number of individuals, and such dynamic changes in number of individuals can only be tolerated by species that possess short or simple life cycles. The annual weeds of arable fields and row crops are usually r-strategists, similarly to the pioneer species of barren surfaces. The r-strategist species are therefore characterized by a high number of progenies (seeds) of relatively low survival chances. Such a species is for example the common chickweed (Figure 10). After the summer drought, the autumn rains trigger the quick sprouting of the late germinating seeds. Plants grow fast and bloom massively until the first autumn frosts kills them. The survival of this species and the germination of seeds next year is ensured by the abundant seed production which accumulates in the soil during the short vegetation period.

Figure 10: Stellaria media – Common chickweed.
Plant populations that follow the K strategy have an initial exponential growth phase which slows down with time (when N= K/2), when fewer and fewer offsprings are born. When the number of individual reaches the environmental carrying capacity (i.e., a critical threshold at which the number of individuals (N) equals the carrying capacity (K) of the ecosystem (N=K), the number of offsprings born will be nil, and the number of individuals in the population stabilizes. These plants therefore try to avoid rapid, mass mortality. This strategy is followed by species that have long life cycles, e.g. trees and perennial herbs. Due to investment in parental care, the new recruits have increased chances to survive.
g) Competitive ability
Populations are competing for environmental resources, and competition occurs in almost every habitat. Its extent depends on the population size, on the carrying capacity and limitations of the environment. Competitive ability is an inherent feature of all populations. There are good competitors with good chances to win and less successful competitors, the “losers”. The latter are usually forced to live in less suitable, abiotically more extreme habitats.
Competition can occur between populations of two different species, which is called interspecific competition, e.g. the competition of different forest trees for ligh and the competition of their roots to reach the groundwater. The roots of woody species can compete with roots of the neighboring herbaceous species.
Another type of competition is the intraspecific competition. When resources are limited, individuals of the same population can compete with each other. For instance the layout of fruit trees in an orchard has to be precisely defined to prevent trees slowing down the growth their neighbours or diminish the fruit production.
A wide variety of inter- and intraspecific competition situations can be found in nature, e.g. in birds intraspecific competition is frequent among the nestlings for the parental care (when the parents cannot provide sufficient food for the young, the weaker nestling often dies).
Forms of intraspecific competition typical to plants, and not found in animal populations, fall within the infraindividual competition types (for details see chapter 7).
As already mentioned in the introduction, plants and plant populations posess unique features, which is why they are treated separatelly from animals in ecology. Plant populations differ not only because of their sedentary life, but also because of their particular body organization and structure.
5. 3. 2. Specific features and characteristics of plant populations
a) Plant body structure
The number of individuals is a specific feature of every population. In plant populations the structure of the plant body determines the number of individuals within a population.
To start with, we have to recall that plants have modular body structure. The module is a repetitive, morphologically well-defined structural subunit of plant individuals. The modules have their own life cycle and can decay without causing the death of the plant individual. For example when an animal loses one of its limbs that will cause serious damage or even death of the individual. In contrast, by cutting off the old and diseased modules, plant individuals can be rejuvenated.
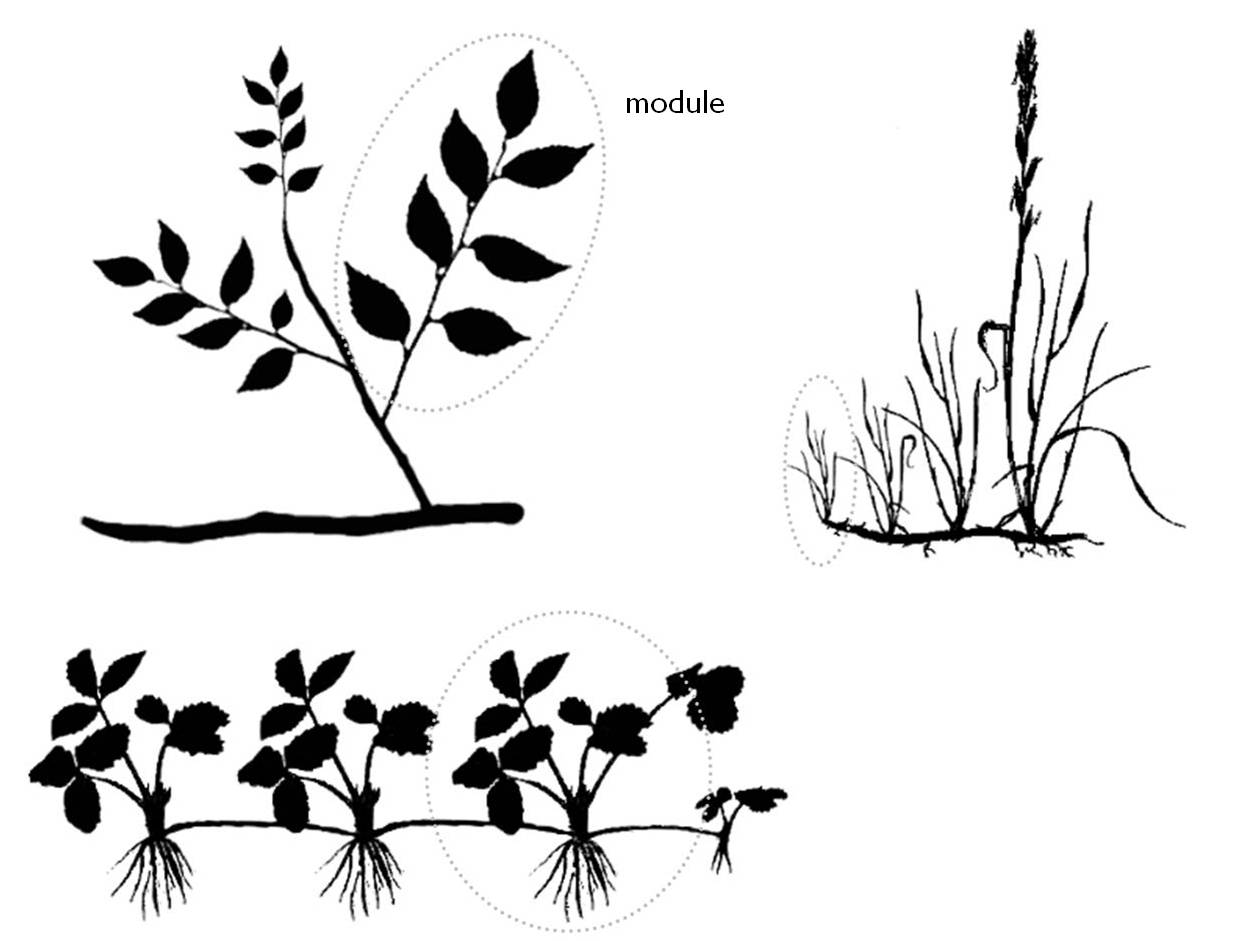
11. Figure: The modular structure of woody-, herbaceous- and clonal species
Plants develop a variable number of modules, but the number of live modules depends always on the suitable environmental conditions. A plant species can respond to the environmental constraints by reducing the number of its modules, but if the resources are abundant the number of modules increases again.
Evaluating the number of individuals in a plant population can sometimes be quite difficult because the individual boundaries are often unclear and we often don’t know how large individuals are (Figure 12).

Figure 12: Cornus sanguinea – Common dogwood, with a large number of modules (clonal structure)
The number of individuals in a plant population can be determined by recording the number of genets (N) i.e., individuals developed from a single seed (germ). So the genet number is equal to the number of zygotes resulted from sexual reproduction. In addition to genets, in plant populations is also informative to record the number of modules (e.g. in root sprouting shrubs) and in this case we are dealing with the number of ramets (η). Therefore the genet of seed plants is an individual that develops from a germ and consists of the total number of ramets. Consequently different ramets of a genet have always the same genetic identity.
The number of individuals of plant populations can therefore be evaluated as Nη.
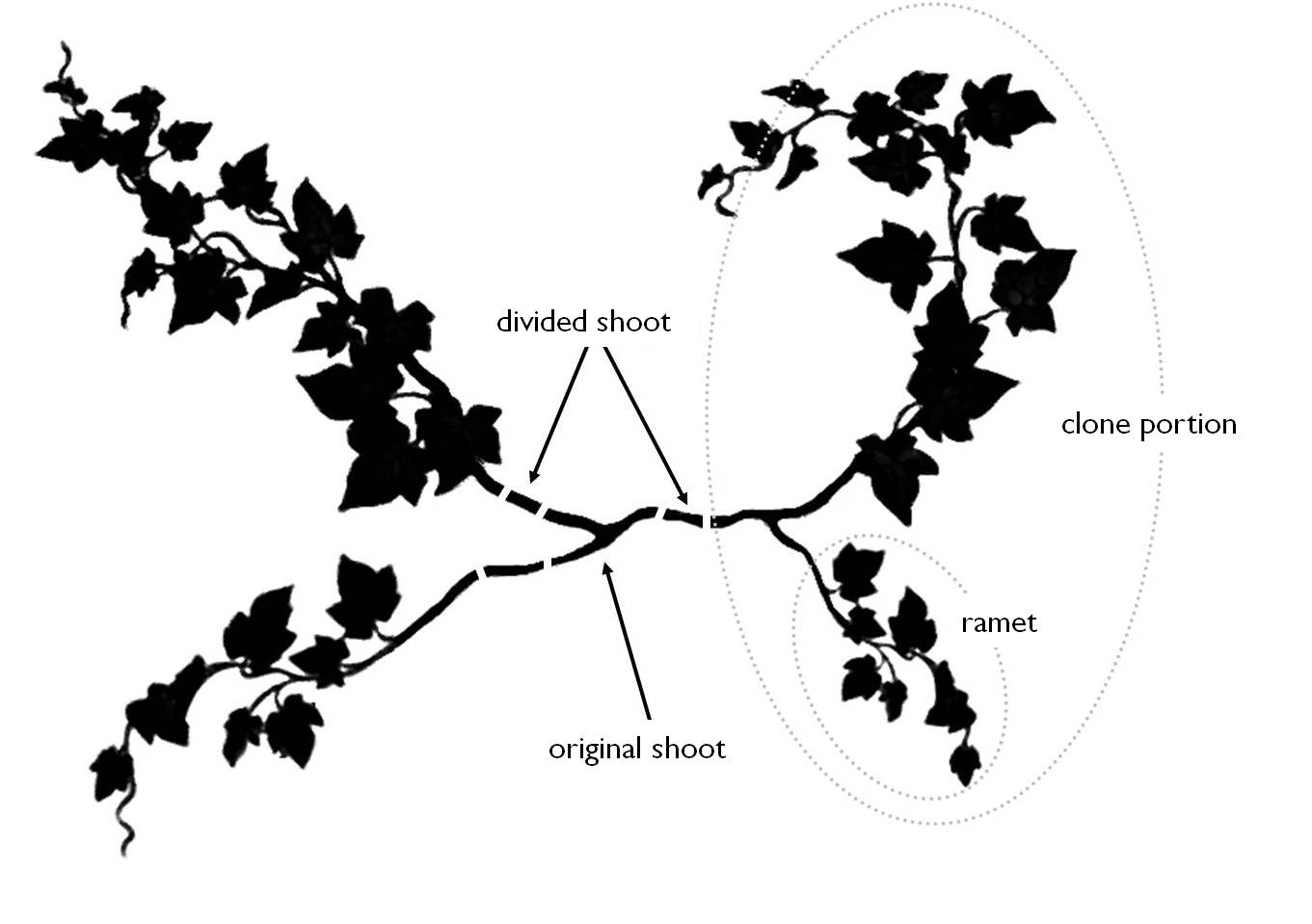
13. Figure: The body structure, the genet-ramet of a plant individual (after van der Maarel)
In horticulture inventorying the number of modules is often sufficient. For example, the number of reproductive shoots (fruiting modules) can be much more informative in a fruit orchard with intensive technology than the number of fruit trees (Figure 14). As well, in vineyards in addition to the number of vine-stocks, the number of reproductive modules is decisive. A high number of vine-stocks is useless if there are too many modules and grapes don’t develop well because of the intraspecific competition. In this case the grapes have to be thinned. By thinning, the number of fruiting modules is maintained at an optimal density.

14. Figure: Intensive apple orchard with dwarf fruit trees that develop a high number of reproductive modules
By vegetative propagation of modules plants can be multiplicated and used for obtaining new individuals of identical phenotypic characteristics (e.g. through stem cuttings or layering) (see Chapter 4 on vegetative propagation). In horticulture, the vegetative propagation is very important in maintaining the quality of the varieties. With these methods we can increase the number of ramets without changing the parental genetic material.
Grafting is a special technique by which two different sets of modules are joined together. Usually modules of one scion plant (stems, buds) are inserted into those of the stock plant. This way we are preserving the features of the sensitive cultivar (scion) while making sure that it stays alive by grafting it on a resistant rootstock. If there is no incompatibility between the inoculated scion and the stock, the modules of the graft will grow vigorously on the rootstock.
b) Spatial structure
Spatial structure can refer to the shape of a plant individual as well as to the geometric pattern of the plant population. The vertical growth of plants is driven by light, and the horizontal growth leads to branching, adventitious rooting and to the development of the clonal structure.
In some plant populations the modular growth is intensive in both directions, which can produce multiple geometric population structures (Figure 15).

15. Figure. The clonal structure of a bamboo species (Bambusa spp.) develops due to the underground branching that produces aboveground woody modules of similar age.
Due to the genet-ramet dynamics, plant populations are able to employ successful survival strategies and place themselves into advantageous spatial positions during competition. The spreading and expansion of some woody weeds is a consequence of their capability to develop complex multidimensional spatial structures (Figure 16).

16. Figure: The invasive vegetative clone of Ailanthus altissima - Tree of heaven at Fóti Somlyó hill (near Budapest, Hungary). (Photo: László Udvardy from 1997)
The clonal structure is common in plant populations, and represents a strategy employed by plants to survive and spread. All members of a clone have identic genetic material, being descendants of a single plant genet (germ of a seed) and all parts are produced only by vegetative multiplication. This asexual form of reproduction that constitutes the fundaments of clonal development is never preceded by genetic recombination.
Because of the clonal strategy many plant species are able to reach very old ages. Investigations on some poplar (Populus tremuloides) individuals (genets) in America revealed that their age is of about 12000 years, which means that the germination of their seeds dates back to the beginning of the Holocene period (Mock et al. 2008, Mitton –Grandt 1996).
Because of various environmental constraints, the number of modules of an individual can vary across a large interval. An adjustable module number is always an important survival strategy. For example the amaranth (also called pigweed; Amaranthus retroflexus) can bloom even when it is a few centimetres high and it is also able to produce viable seeds. But if the environmental conditions are suitable, an individual may grow meter heigh with hundreds of modules that produce a huge number of seeds. A high seed production in a year can fill up the soil seed bank for dozens of years (Figure 17).

17. Figure : Different sizes of flowering Amaranthus retroflexus (red-root amaranth or red-root pigweed) individuals
c) Life strategies
The behaviour of plant populations can be described by different types of life strategies. Grime’s (1979) ecological life strategies are also related to the population regulation.
Improving the r- and K-regulation strategies and by considering environmental extremes in addition to competition, Grime suggested three fundamental life strategies for plants:
S – stress-tolerant species:
- inhabit extreme habitats
- have high adaptive capacity
- have low productivity
This life strategy is typical for most of the succulent taxa e.g. for Sempervivum (liveforever) and Sedum (stonecrop) species.
C – competitor species :
- very strong vegetative propagation ability
- are often clonal
- can be invasive by having a multidimensional spatial structure (!) e.g. Acer negundo (Boxelder maple), Cirsium arvense (Creeping thistle)
R – ruderal species:
- annual or biennial plant species
- produce a large number of seeds, which are viable for a long time
- live in pioneer, disturbed or human-made habitats e.g. Amaranthus (amaranths), Chenopodium (goosefoots)
Grime described also transient strategy types, which can be simply illustrated with a triangle (Figure 18). Environmental characteristics that prevail in a given habitat often determine the best strategy for plants and their populations.
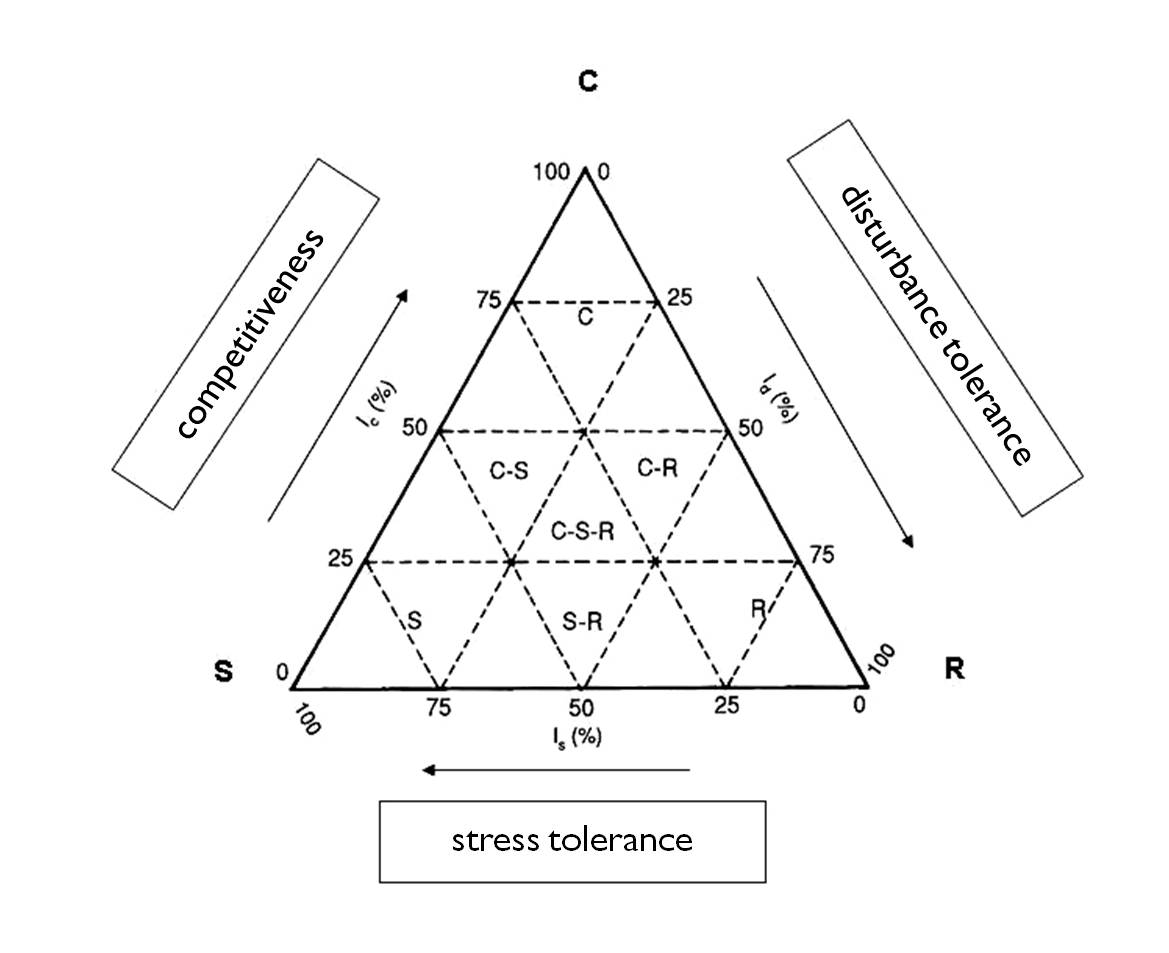
18. Figure: Grime’s life strategies
d) Immobility
A very obvious feature of higher plants is their sedentary life, which affects their growth and spreading strategy throughout their entire lifetime.
e) Plant life cycle
John Harper (plant population ecologist) created a four-phase model of plants’ life cycle (1977). According to this model the life cycle of a plant individual consists of four important sections: seed rain, seed bank, recruitment and individual modular growth. In addition to the final stage visible to us, the other three phases are equally important for the survival of plant populations (Figure 19).
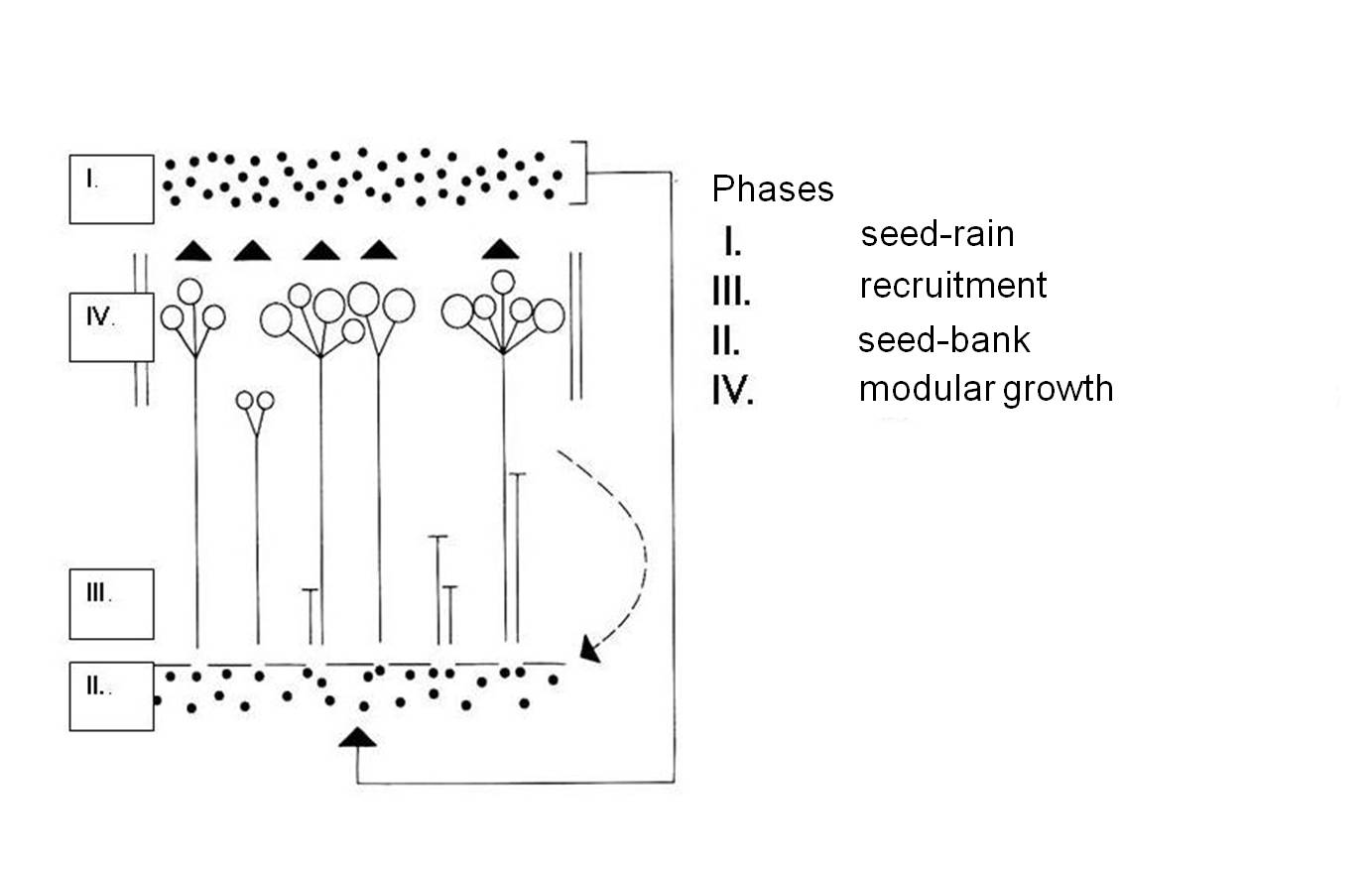
19. Figure. A model of plant’s life cycle (after John Harper)
- Seed rain or inoculum
Seed rain means the dispersal of all propagative plant organs, the propagules - propagula. In a wider sense it presumes the propagation of any generative or vegetative plant organs which inoculate the soil.
A population either “pours” the seed rain locally, ensuring the survival of the population in that particular habitat, or scatters the seeds at longer distances, ensuring the colonization of new localities.
The intensity of seed rain depends on the seed yield, seed dispersal type, plant height, distance from the seed source, dispersal ability of propagules and finally on the quality and morphological characteristics of the dispersal organs (Figure 20).

20. Figure. Different type of fruits and seeds. A: Tilia (linden) – wing like bract; B: Acer (maple) – schizocarp samara; C: Ulmus (elm) – samara with oval wing; D: Clematis (clematis) – achene with tail like, feathery pistil; E: Taraxacum (dandelion) – cypsela attached to pappus F: Pinus (pine) – winged seeds; G: Asclepias (milkweed) – seeds are comose at the apex; H: Arctium (burdock) – cypselae disperse together within the capitula provided with hooked bracts; I: Harpagophytum (devil's claw) – hooked capsule.
The seed rain is also influenced by environmental conditions and by habitat characteristics such as the weather (wind strength and precipitation), topography, the onset of grazing, migratory animals, etc.
The timing of the seed rain is very important. Plant populations in special habitats need to time their seed rain for the best period when the chance of survival and spread of propagules is the highest. For example species of dry habitats and weeds of fallows disperse their seeds at the beginning of the wet season, whereas some conifers (e.g. Pinus contorta) in the permafrost regions open their cones and release seeds only in the event of spontaneous forest fires, when the soil becomes warmer, suitable for seed germination.
- Seed bank
The seeds are usually dormant for shorter or longer periods before germination (dormancy). The onset and the end of dormancy are regulated mainly by physiological functions, but they are also strongly influenced by environmental conditions. Seeds are being spread during their dormant phase and therefore they can accumulate in the soil seedbank. The seedbank of a habitat may contain seeds released by the population of that particular habitat but propagules from other more distanced sources as well. Usually the species number and the number of genets in the seed bank are not identical to the species-, and individual numbers of the above-ground populations.
As a result, the species list of the soil seed bank can be considered as a hidden flora of an area. Two explanations exist for when a species is not observed in a given habitat: it is either not available in the seed bank or its propagules are not able to activate.
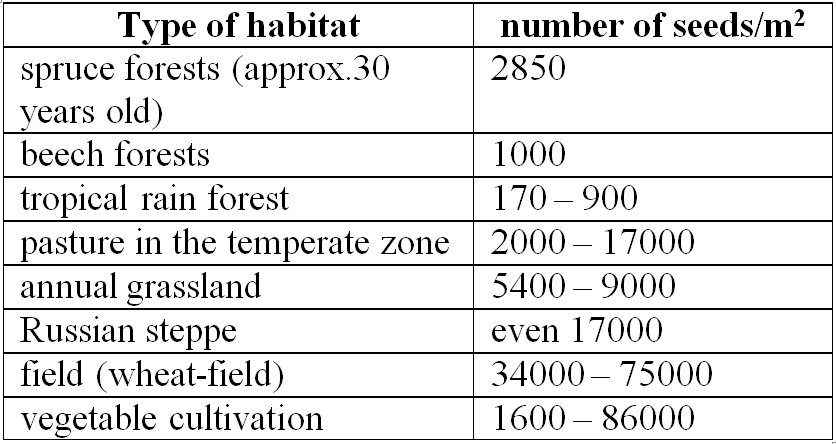
When assessing the plant diversity of an area, the seed bank should also be taken into account. Soils can store large amounts of seeds. Gardeners that start cultivating in places that have been previously abandoned for several years, usually have to deal with a massive amount of weed propagules accumulated over the years.
2. Table The size of the seed bank (number of seeds) in different habitat types
The hidden life of certain terrestrial orchid species can be explained by their ability to survive underground (as vegetative propagules) for longer periods of time. In dry years, when the chance of producing flowers and seeds is very low, orchids do not develop aboveground organs despite being represented by a viable population in the soil.
Seeds of some species have very short dormancy, whereas others may completely lack seed dormancy and are usually missing from the soil seed bank. E.g. seeds of Salix (willows) and Populus (poplars) species will either germinate when they are released, or will decay very soon. The viability of seeds may vary on large scale. Some seeds can maintain their germination ability for only a few years, but 3000 years old lotus ( Nelumbo nucifera) seeds found in a lake near Tokyo were also perfectly viable. The preservation of germination ability also depends on the environmental conditions of the given habitat.
- Recruitment
The recruitment from seed bank is an important event. Not all propagules or seeds present in the soil seed bank will grow to become new plants. Some seeds fail to germinate at the onset of the suitable germination conditions, others are consumed by rodents or can be the victims of fungal infections or of other pathogens. Finally, the seeds to successfully survive all these risks have to experience specific impulses (e.g., environmental factors) which will trigger their germination and the input of new recruits to the system. The parental population is often able to regulate the recruitment. For example, if there are older trees in a forest, the suppression of the new recruitment is almost guaranteed.
However, old trees can also enhance recruitment by the „nurse tree” effect, when the development of the recruits is facilitated by the faster- growing or older individual, which protects the seedlings from strong radiation, from the drying effect of the winds or from pests.
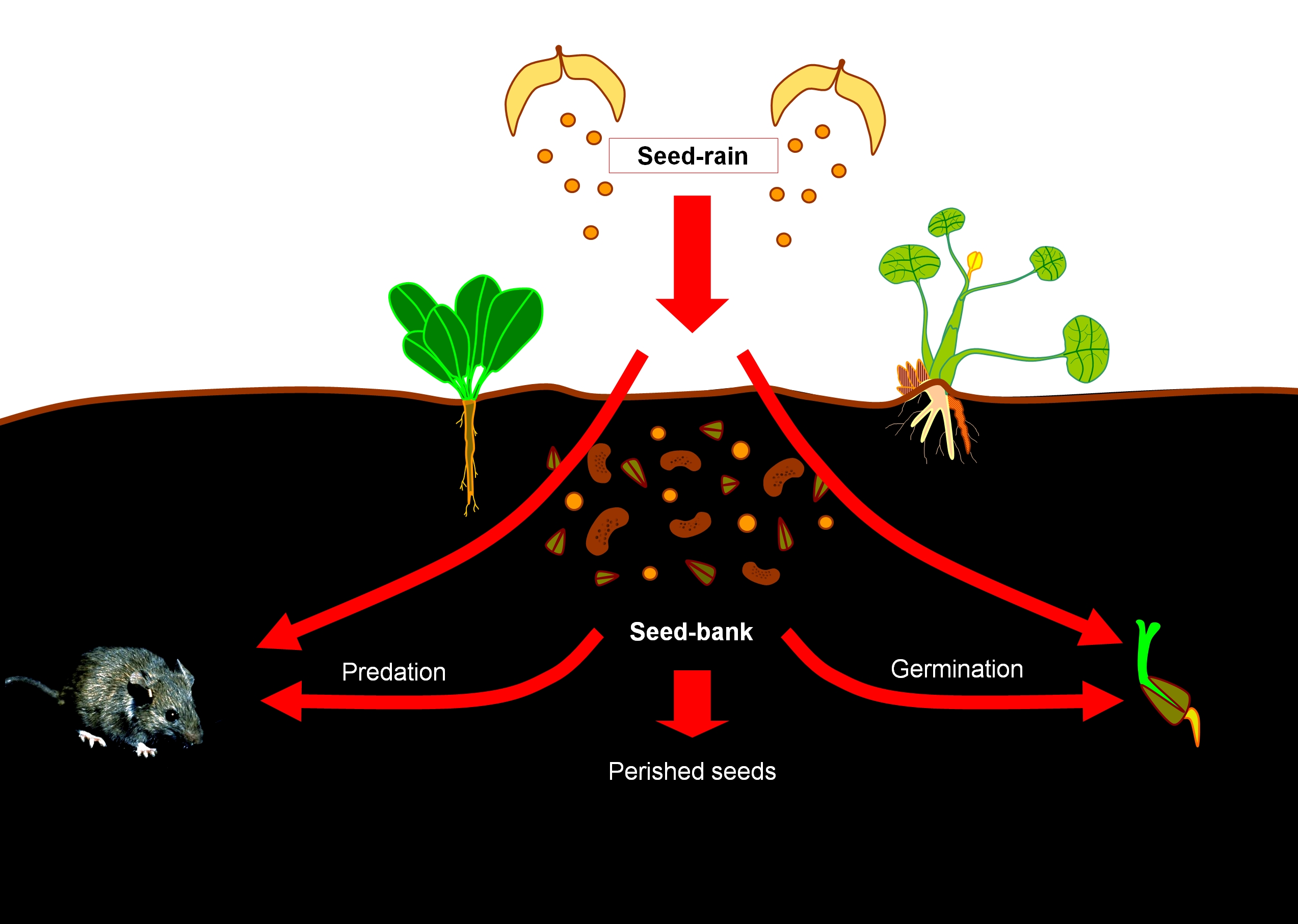
21. Figure. The fate of the soil seed bank
- Individual modular growth
As mentioned above, the germination and growth of individuals in a plant population will sooner or later lead to a population density which makes competition inevitable.
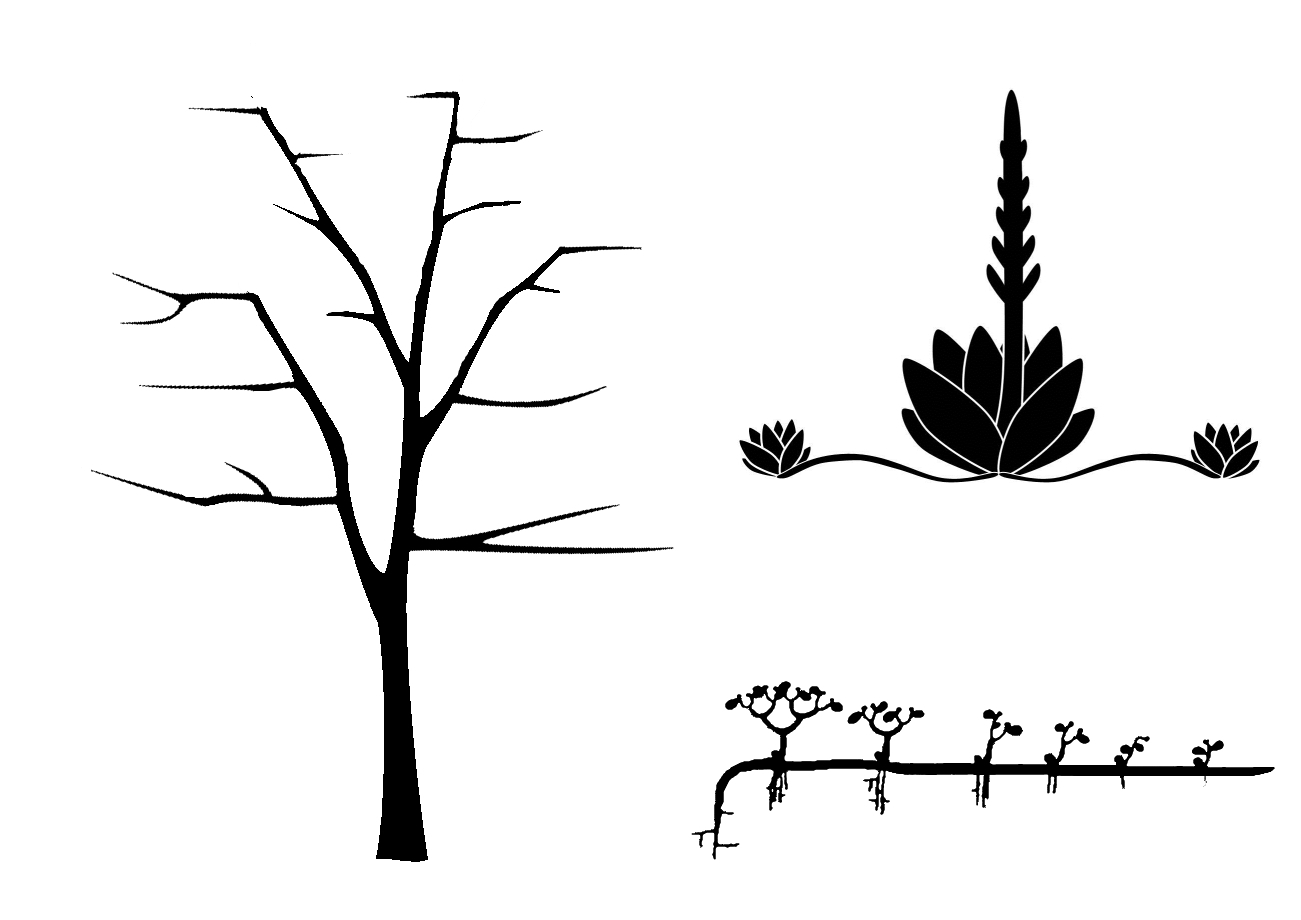
22. Figure: Growth and spatial structure of individuals with different modular structures
References
Borhidi A. 2009: A növényvilág evolúciója és a darwini fejlődéselmélet. Magyar Tudomány. 9.
Cristea, V., Denaeyer, S. 2004: De la biodiversitate la OGM-uri. Ed. Eikon. Cluj- Napoca.
Euforgen adatbázis: http://www.euforgen.org/
Grime, J. P. 1977: Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 111:1169-1194.
Harper, J. L. 1977: The Population Biology of Plants. Academic Press.
Hewitt, g. M. 2001: Speciation, hybrid zones and phylogeography, or seeing genes in space and time. Molecular Ecology. 10: 537–549.
Láng I., Bedő Z., Csete L. (szerk.) 2003: Növény, állat, élőhely. Magyar Tudománytár. Kossuth Kiadó. Budapest.
Mitton J. B., Grant, M.C. 1996: Genetic variation and the natural history of quaking aspen.
BioScience 46: 25–31.
Mock, K. E., Rowe, C. A., Hooten, M. B., DeWoody, J., Hipkins, V. D. 2008: Clonal dynamics in western North American aspen (Populus tremuloides). Molecular Ecology 17: 4827–4844.
Mora, C., Tittensor, D. P., Adl, S., Simpson, A. G. B., Worm, B. 2011: How Many Species Are There on Earth and in the Ocean? PLoS Biol 9 (8): e1001127. doi:10.1371/journal.pbio.1001127
Standovár T., Primack, R. B. 1998: A természetvédelmi biológia alapjai. Nemzeti Tankönyvkiadó. Budapest.
van der Maarel, E. 2005: Vegetation ecology. Blackwell Publishing.
Vida G. 1996: Luxus vagy nélkülözhetetlen – Az élőlények sokfélesége. Természetbúvár, 51(4): 10-12.

