Plant tissues (Histology)
Author: Zsolt Erős-Honti
2.1. Concept and evolution of plant tissues
The term tissue refers to a specialized group of cells of the same origin and similar structure and hence similar function. Due to the characteristic way of plant development (i.e. the special process and levels of cell differentiation) the cells of each plant tissue can be traced back to a certain group of cells (even single cells). Consequently, based on their ontogeny, differentiated plant cells can be classified into a hierarchical system. Cells of a given function (e.g. those of the stomata) compose simple tissues, the members of which – usually – derive from a single cell of the respective meristem. Of the simple tissues so-called complex tissues (e.g. epidermis, xylem) are composed. Group of tissues serving similar purposes, and so having similar structure and similar positioning within the plant compose the tissue systems (e.g. the epidermis, rhizodermis and periderm comprise the dermal tissue system – in details see later!).
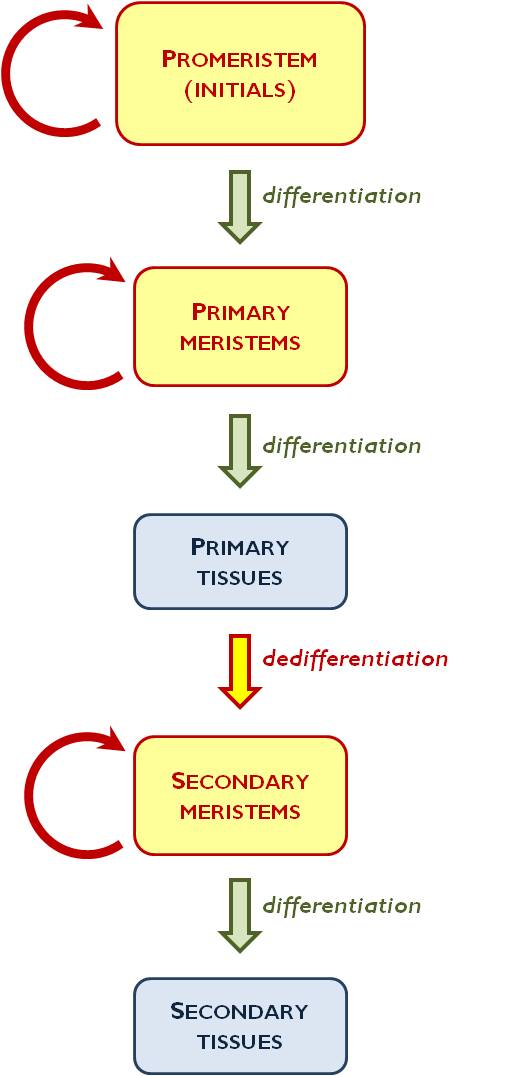
Figure 1. Differentiation of plant tissues
During the evolution of plants, invasion of terrestrial habitats demanded for the development of the tissues. Only those plant forms could survive in the new environment, the cells of which divided the labour and thus had differing structures. This degree of labour division resulted the differentiation of the tissues. (Similarly to the vascular plants, bryophytes and hepatophytes also invaded terrestrial habitats, which induced cell differentiation in case of these plants, too. However, the level of their differentiation could not cope with that of the ancient ferns and so no further plant forms could evolve from the mosses: they comprise a mere side branch on the plant’s tree of life.)
Among the recent plant groups, only ferns, gymnosperms and angiosperms (the vascular plants) have real tissues.
2.2. Categories and differentiation of the plant tissues
Plant tissues can be divided into two main categories: meristems and differentiated tissues. Owing to their continuous mitotic divisions, meristems’ task is to increase the cell number of the plant organism. From the two derivatives of a meristematic cell one keeps the level of differentiation while the other starts to differentiate. This means either a more differentiated meristem or the daughter cell ceases to divide and becomes a differentiated tissue cell (i.e. it comes out of the cell cycle into the so-called G0-state).
Sometimes a differentiated tissue cell can regain its mitotic activity (as a normal process of the plant body’s development), yet it is no longer a differentiated tissue cell, but turns into a meristematic cell again – this is the process of dedifferentiation.
The least differentiated types of meristematic cells are the initials (promeristem) (Figure 1.). Their derivatives can still divide by mitoses; they comprise the primary meristems. Primary meristem cells produce completely differentiated primary tissue. If it is required by the genetic program of the plant’s development (e.g. in case of the secondary thickening of the root or the stem), primary tissue cells regain their mitotic activity and turn into secondary meristems (e.g. phellogen). The activity of secondary meristems again results differentiated tissues; these are the secondary tissues (e.g. periderm).
Differentiated tissues can be classified into three tissue systems. The ground body of the plant is composed of the ground tissue system, which may serve manifold functions (photosynthesis, storage, secretion). Dermal tissue system isolates and protects the plant body from its environment, yet in the same time it provides the communication between the inner parts of the plant and the surrounding. The vascular (conductive) tissue system conducts plant nutrients (water and minerals) and organic compounds (sugars, amino acids, certain hormones) within the plant.
2.3. Meristems
In case of the animal body, the majority of the cells is capable of mitosis. To the contrary, in plants new cells are produced only at certain parts of the body, within the meristems. All the plant cells derive from these tissues, and only later – during the process of differentiation – gain their purposed function. These cells are usually small and isodiametric (i.e. they are bordered by polygonal cell walls of more-or-less the similar extension in all dimensions). Their cell nucleus is relatively large in proportion to the cytoplasm and also to the total cell volume. Meristematic cells are typically undifferentiated ones: their cell wall is a thin, extensible primary cell wall, there are several small vacuoles within the cytoplams, and (if there is any plastid within them) they have proplastids.
Meristems can be classified on several different bases.
-
According to origin and degree of differentiation:
-
Promeristem : the least differentiated meristem type, composed of initial cells.
-
Primary meristem : the slightly differentiated derivative of the promeristem. It produces primary tissues.
-
Secondary meristem : a meristem formed by the dedifferentiation of primary (differentiated) tissues. It produces secondary tissues.
-
According to position:
-
Apical meristems : dividing tissues situated at the poles of the plant body, accomplishing the longitudinal growth. Their two types are the root tip and the shoot tip.
-
Intercalary meristems : meristems situated between differentiated tissues causing secondary lengthening of the plant body. They typically occur in the nodes of the grass stem, in the nodes of several rosette plants or at the base of the grass leaves.
-
Lateral meristems : meristems causing the secondary thickening of the plant body, situated on the periphery of the organs. Two instances are the vascular cambium (producing secondary vascular and ground tissues) and the phellogen (producing the periderm).
Primary and secondary meristems establishing the certain organs will be discussed later, in the respective chapters on organography.
2.4. Dermal tissue system
Plants are covered by the elements of the dermal tissue system: these tissues are responsible for the integrity of the plant body. Besides, positioned on the surfaces of the organs, dermal tissues serve the interconnection between the interior of the plant and the outer environment. Consequently, the main functions of these tissues are: protection against mechanical, chemical and biological (pathogen) impacts, nutrient absorption, gas exchange and transpiration. Additionally, dermal tissue elements may play a role in accumulation, as well as in secretion.
Composing differentiated tissues, the cells of the dermal tissue system cease dividing; they have thickened secondary cell wall and a large vacuole. Their cell nucleus is relatively small in proportion to the cell volume. Serving the function of protection, connections between the cells are quite strong. There are no intercellular spaces. This function is usually also enhanced by the fact that the neighbouring cells connect to each other by a large, undulating cell wall (the cells are similar to the tiles of a puzzle game). Connections between the epidermal cells are stronger than those between the epidermal cells and the underlying tissues, thus usually the dermal layer can be easily pulled off from the organ surface (this is also a tissue preparation technique, called peeling). Cells are large, flat and usually form one cell layer on the surface. They contain chloroplasts only exceptionally (e.g. in the epidermis of the thin, submerse leaves and in the guard cells of stomata). (However, it is notable that storage leucoplast are rather frequent in them.) Cell walls facing outside are often thickened, owing to the apposition of cutin, suberin and/or wax layers.
2.4.1. Rhizodermis: the primary dermal tissue of the root
One of the basic functions of the root is the absorption of nutrients (water and ions) from the soil. This role is served in angiosperms by special structures of the primary dermal tissue of the root, called root hairs. Root hairs occur in the zone of absorption (see later!) of the thinnest root branches (fine roots). Root hairs are not individual cells but mere finger-like appendages of the rhizodermal cells. Root hairs are not separated by cell wall from the adjacent rhizodermal cell. Root hairs admirably increase the absorptive surface of the root.
In addition to the presence of root hairs, further differences between rhizodermis and epidermis (discussed below) are the – usual – lack of stomata, epidermal hairs and cuticle.
2.4.2. Epidermis: the primary dermal tissue of the shoot
Most of the functions discussed in the introduction part on dermal tissues are to be linked to the epidermis, the primary dermal tissue of the shoot. The stomata of the shoot (on both the stem and the leaves) serve the function of gas exchange and transpiration. Epidermis is often covered by a thick cuticle and bear epidermal hairs, both protecting the plant body. Epidermal glands and glandular hairs are responsible for the function of secretion.
2.4.2.1. Stomata
Water vapour, CO2 produced by respiration and O2 released in photosynthesis leave the plant body through controlled openings of the epidermis, called stomata (sing. stoma). Similarly, here takes up the plant the CO2 required for the photosynthesis and O 2 for respiration, as well. Since the functions of stomata are served by several cells of different structure, more reasonable is to use the term ‘stomatal complex’ for them.
Stomatal complexes always contain two guard cells surrounding the regulable opening of the complex, the stomatal pore ( stomatal aperture). The pore leads the air into a larger intercellular chamber within the ground tissue, called sub-stomatal cavity. The guard cells are mainly kidney-shaped, yet in the family Poaceae (grasses) they resemble dumb-bells. A cytological characteristic of the guard cells is the presence of chloroplasts. Their cell wall is unevenly thickened: around the stomatal pore it is much thicker than at other surfaces. In addition, cuticle layer is also deposited on the guard cells, increased in thickness near the aperture. Moreover, around the outer opening of the stomatal pore, a cuticle ridge aids the complete closure of the stoma. (In case of some species, on the inner surface of the pore another ridge, facing the sub-stomatal cavity, is observable.)
In most genera, guard cells of the stomata are surrounded by ordinary epidermal cells; this is called the anomocytic stoma (e.g. Cyclamen , Ranunculus, Cornus, Iris) (Figure 2). In other cases, stomata serve their function with the aid of cells different from the other epidermal cells (subsidiary cells). If the stomatal complex contains two subsidiary cells, lying parallel with the guard cells, the complex is called paracytic (e.g. in Rubiaceae or Magnoliaceae). If the two subsidiary cells lie perpendicular to the guard cells, the stomatal complex is diacytic (e.g. Lamiaceae or Caryophyllaceae). Anisocytic stomatal complex contains 3-5 subsidiary cells, often of different size (e.g. in the genera Begonia and Sedum or in the family Brassicaceae). Should several, radially arranged subsidiary cells encircle the guard cells, the stoma is called actinocytic (e.g. Anacardiaceae). Subsidiary cells surround the stoma in a ring-like manner in case of the cyclocytic stoma (e.g. some species of the genera Austrobaileya and Baccharis). In addition to the listed basic types, there are several other possible arrangements of the subsidiary cells (e.g. hexacytic stomatal complex with six subsidiary cells or the heliocytic complex with several encircling subsidiary cells of different size).
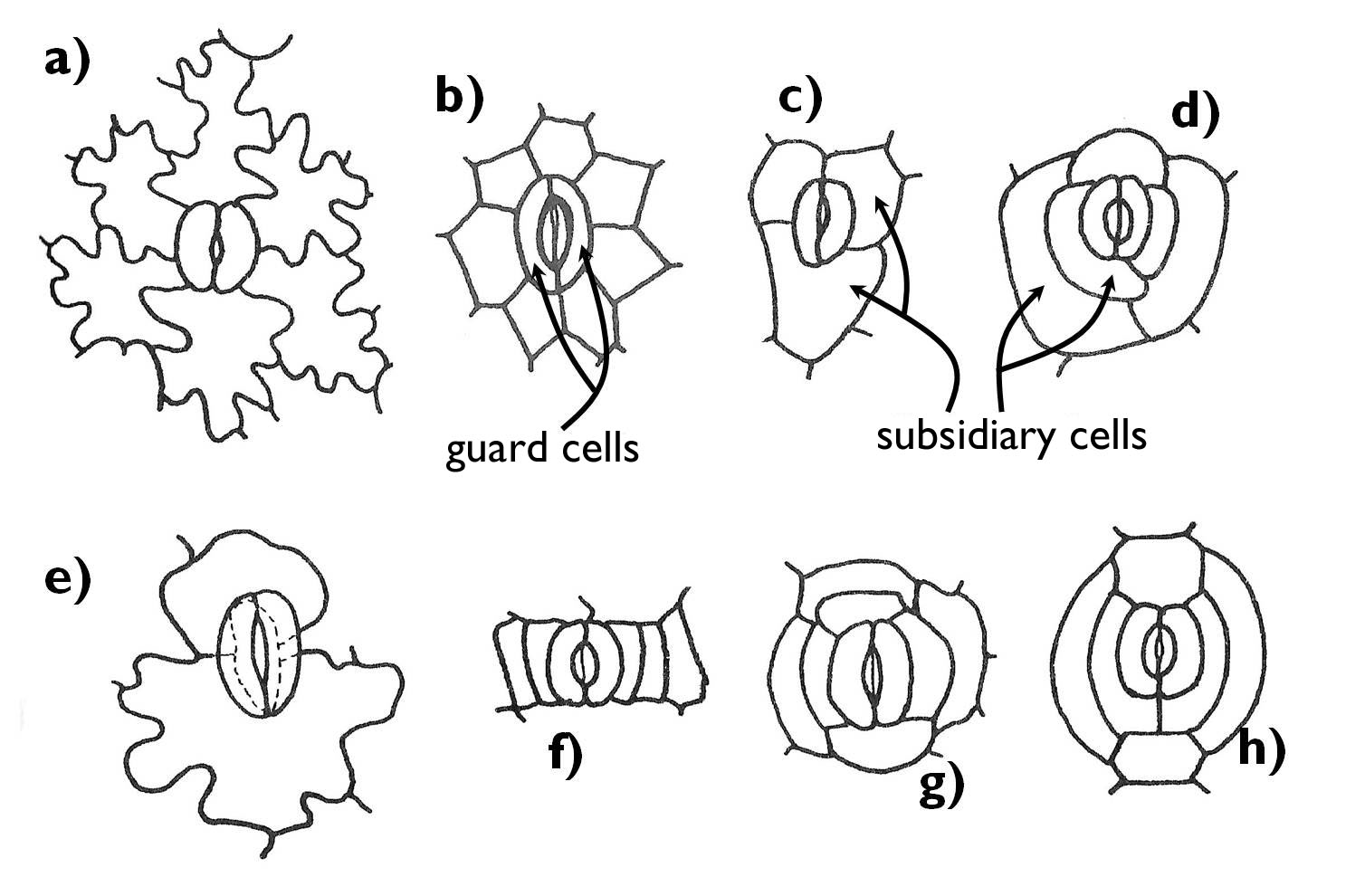
Figure 2. The main types of stomatal complexes.
a) anomocytic; b) actinocytic; c) anisocytic;
d) heliocytic; e) diacytic; f) paracytic; g) ciclocytic; h) hexacytic.
(Based on Gyurján)
2.4.2.2. Cuticle
A typical feature of the dermal tissue covering the shoot is the cuticle layer of the epidermal cells. It is composed of two main groups of compounds: the hydrophobic matrix of the cuticle called cutin, and different waxes. Waxes may form a distinct layer on the surface of the cuticle (epicuticular waxes), but a significant proportion is embedded into the cutin matrix (intracuticular or cuticular waxes).
Cuticle has an obvious stratified structure. The outermost layer is composed of the epicuticular waxes (wax layer), which cause a glaucous surface with a velvet-like shine. Its principal purpose is to reduce the water-loss through the cuticle (cuticular transpiration). Compounds of the wax layer reach the surface through the minuscule cracks of the cuticle. They either form a continuous, amorphous layer, or precipitate in a more-or-less crystalline form, in the shapes of fibres, small rods or scales.
The principal role of the cuticle is protection. Shoots with thick cuticle are thick and rigid. They are more resistant to mechanical effects, even the chewing of the herbivorous insects. Being water repellent, cuticle prevents soaking, yet it also impedes the water-loss. The cuticle may also protect against certain chemical impacts.
Cuticle may serve as a barrier against some biological threats. In case of pathogen (fungal, bacterial) attacks, when the parasite is to penetrate into the shoot through the epidermis, at first its degrading enzymes begin to break down the compounds of the cuticle. From the decaying cuticle, substances hindering the further incursion (even growth) of the pathogen may be released. Other compounds of the degrading cuticle serve as signals (hormone-like substances) and activate the defensive system of the plant.
2.4.2.3. Epidermal appendages
In contrast with the primary dermal tissue of the root, epidermis may possess real plant hairs (trichomes) that are isolated from the ordinary epidermal cells by cell wall (Figure 3). The structure and function of the trichomes are manifold.
In the most general case, the epidermal cells bear simple protrusions. These papillae cannot be regarded as trichomes. These structures refract the light beams and thus gives the organs a velvety shine – they are frequently found on the perianth (e.g. on the corolla of Viola or Saintpaulia species).
The epidermis chiefly possesses uni- or multicellular trichomes. The latter ones have a foot or basal cell in the plane of the epidermis. Stellate and squamiform hairs (or scales) have several radial upper cells derive from the multicellular base (e.g. on the shoot of Elaeagnus angustifolia). (Stellate hairs have far less radiating upper cells and these cells are free at least to the half of their length, while the upper cells of the squamiform hairs are fused almost to their end.) Quite often the axis of the multicellular trichomes is branching. These are the so-called candelabriform hairs (e.g. Lavandula angustifolia, Verbascum phlomoides). Cells of the trichomes frequently die early as a result of apoptosis, then the cell lumen is filled with air. In these cases the hair becomes white, giving a silvery shine to the covered organ.
The function of trichomes is manifold. By forming a mechanical barrier they can protect against the chewing of herbivorous insects. Due to the air trapped among the hairs, they may form a heat isolator layer on the plant surface – for example in case of alpine plants (e.g. Leontopodium alpinum). At the same time, a coat of dead, light trichomes reflects an admirable proportion of the light reaching the organ surface (i.e. increases the albedo), what may be adventitious in hot, sunny habitats, since it protects the plant against overheating (e.g. Cerastium tomentosum, native in the Mediterranean). Similar is the background of the protective effect of plant hairs against light (and also UV) stress in the intensely illuminated areas (e.g. in the highlands), where the proportion of the direct light within the solar radiation is high.
The air trapped among the epidermal hairs is quickly saturated with released water vapour, significantly decreasing the rate of transpiration. Thus trichomes are beneficial also for plants living in arid environments. Nevertheless, some bromeliads (Bromeliaceae) have water absorptive scale hairs on the adaxial epidermis of their leaves that form a rosette. By their special trichomes these plants can take up the water from the cistern formed by the leaves (or directly from the falling precipitation or even from the surrounding air of high water vapour content).
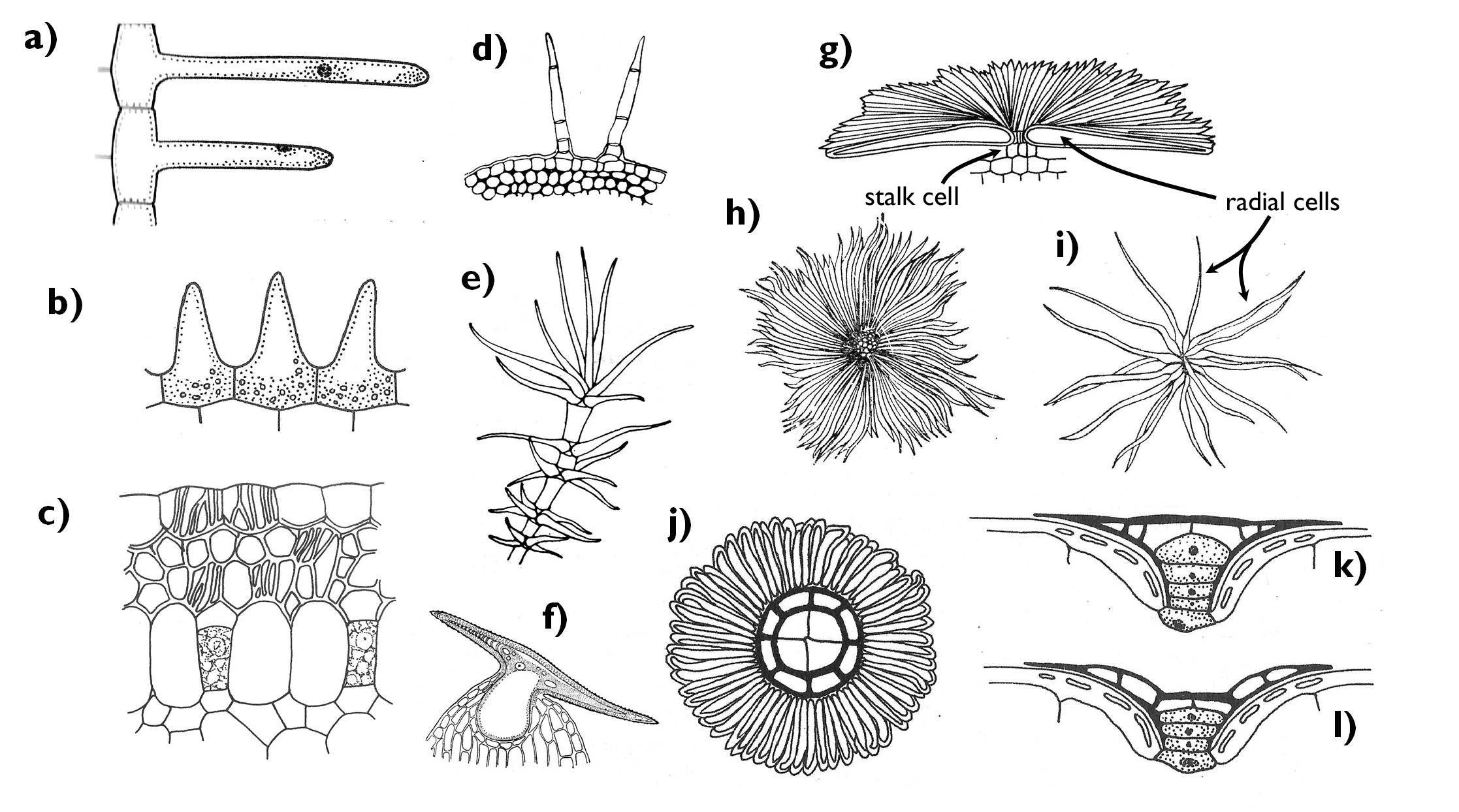
Figure 3. Epidermal appendages. a) root hairs; b) papillae; c) velamen radicum; d) multicellular and simple trichomes; e) candelabriform tirchome (Verbascum); f) hooked trichome (Humulus); g) squamiform trichome, side-view; h) squamiform trichome, plan-view (Elaeagnus); i) stellate trichome (Elaeagnus); j-l) water absorptive scale hair, plan view (j), filled with water (k) and empty (l) (Bromeliaceae).
(Based on a-b: Jacob et al., c: Gyurján, d-f: Kárpáti, g: Jacob et al., h-i: Sárkány and Szalai , j-l: Jacob et al.)
By the alteration of the structure and function, other hair types have also evolved. Thick walled, rigid trichomes are the bristle hairs (they are general features of the families Boraginaceae and Cucurbitaceae), which increase protection against the herbivorous arthropods. Hooked trichomes, strengthened by an inner crystal, are to be found on the shoot of the hop (Humulus lupulus): these clinging hairs help to fasten the stem of the creeper on its support. (Owing to their inner crystals, these hairs are also known as cystolith hairs.) Hairs may also occur on the outer surface of the seeds (tuft hairs), what may enable the wind dispersal (anemochory) (e.g. on the seeds of the genera Salix, Populus, Gossypium).
A special, multilayered epidermis covers the aerial root of epiphytes living in the rainforests. This so-called velamen radicum is composed of dead cells. Due to the peculiar, channelled secondary thickenings of these cells composing a labyrinth-like system of capillaries, velamen is capable of absorbing the water from the vapour-saturated air of the tropical forests, or also from the draining precipitation.
2.4.2.4. Secretion in the epidermis: the outer secretory structures
The term ‘secretion’ refers to a complex process of the cell, when it expels certain substances from its living parts (the protoplast), or it separates them in an isolated compartment. Actually, all plant cells have the possibility to produce compounds that may be applied later either within the cell, in the extracellular spaces or even outside the plant body. However, in plant anatomy the function of secretion is basically assigned to special secretory tissue elements. Such elements are found in the epidermis and also within the ground tissues. Based on origin, endogenous and exogenous secretory structures are distinguished. The previous ones are also called ‘plant glands’.
Cells of the glands either produce the secreted substances themselves (glandular hairs, osmophores) or the secreted compounds simply leave the plant body via these structures (e.g. hydathodes, nectaries, salt glands). The secreted material can be either hydrophilic (e.g. mucilage, nectar, salts) or hydrophobic (e.g. volatile oils). All secretory cells have dense cytoplasms due to the increased number of certain organelles. (E.g. mucilage cells have several Golgi bodies, while those producing lipids have an increased surface of SER.)
Species of several eudicot (Rosopsida) families have epidermal secretory appendages, glandular hairs (Figure 4). The common characteristic of these various anatomical structures is that their secreted material accumulates under the outer cuticle of the cells and gets outside when the cuticle ruptures. In the anticlinal cell walls of the secretory cells suberin or cutin is deposited so that to block the apoplastic transport (see later!) within the cell wall. Secretory cells always differ from the ordinary epidermal cells. The anatomy of the glands is manifold: they may be uni- or multicellular, stalked or sessile (for example in the genus Pelargonium all these types can be observed). In the labiate family (Lamiaceae) multicellular glands (peltate trichomes) stands in the plane of the epidermis. These glands are composed of a larger basal (stalk) cell and eight secretory cells above, producing volatile oils. In addition to oils, glandular trichomes may secrete other terpenoids (e.g. resin) or flavonoids. The aims of the process of secretion are various: repelling herbivors, attracting pollinators or due to their possible sticky nature, they may help fruit dispersal.
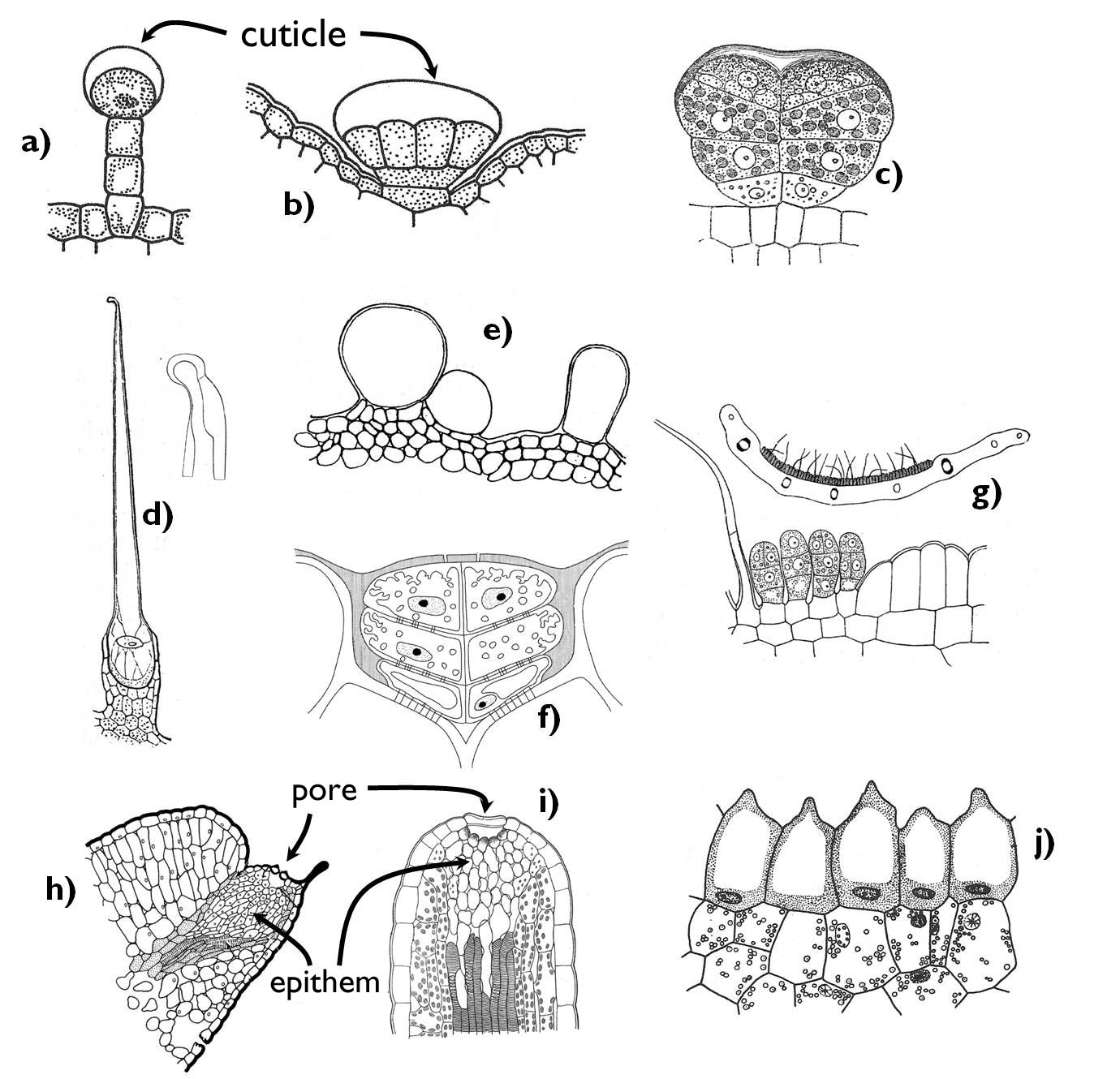
Figure 4. Epidermal secretory structures. a) glandular trichomes (Pelargonium); b-c) peltate glandular glands (b: Lamiaceae, c: Asteraceae); d) stinging hairs; e) water storage trichomes ( Tetragonia tetragonoides) f) salt glands; g) nectaries; h-i) hydatodes; j) osmophore.
(Based on: a-b: Jacob et al., c-d: Haberlandt, e: Kárpáti nyomán, f: Beck, g: Haberlandt, h: Evert and Eichhorn, i: Haberlandt, j: Haraszty)
Outer secretory structures releasing a fluid with high sugar content are the various nectaries. Two main types are the intrafloral (floral) nectaries being within the flowers and the extrafloral nectaries that are located outside the flower (e.g. on the pedicel or the flower stalk). The former ones produce food for the pollinators, whilst the extrafloral ones attract chiefly insects (usu. ants) that may protect the plant against the herbivors1. The position of intrafloral nectaries may be manifold. They may establish on the inner wall of the receptacle, in a cushion-like form around the ovary or it can cover the upper surface of the ovary. Besides, certain floral leaves (e.g. sepals or stamens) may also reduce and turn into nectarines. The secretory tissue of the nectary may develop as a simple, modified epidermis (sometimes bearing uni- or multicellular glandular trichomes, as in the genus Tilia). More frequently, a glandular ground tissue of one or several cell layers (‘nectariferous tissue’) produces the sugary fluid, which gets to the surface through the modified stomata or the outer cracks or minuscule openings of the outer surface of the epidermis. In addition to sugars (sucrose, glucose and fructose), nectar may contain a low amount of amino acids, other organic acids, proteins (chiefly enzymes), lipids, minerals, phosphates, alkaloids, phenoloids and antioxidants. Its components mainly derive from the phloem elements ending near the nectary; however, the composition of the nectar slightly changes within the nectariferous tissue.
Special external secretory structures can be found on the leaves of carnivorous (insect trapping) plants. In different plant genera, the morphology of the trap leaves may be rather different and thus the glandular structures may also be various. They have three main types: nectar producing glands with the purpose of attracting the insects, mucilage glands to cover the trap surface with a slimy discharge (thus preventing the rescue of the prey) and glands producing the digestive enzymes. In case of some species, mucilage and enzymes are produced by the same secretory elements (e.g. that in the head of the tentacles of the sun-dews (Drosera)). A typical feature of the glands of the carnivorous plants is their ability to absorb the digested compounds in addition to their secretory function.
2.4.2.5. Further cell types and additional functions of the epidermis
Beside the above mentioned cell types, the dermal tissue covering the shoot may contain other cells that serve the adaptation to certain environmental conditions.
For instance, the epidermis on the leaves of grasses (Poaceae) has various different cells. Here the epidermal cells are traditionally called ‘ long cells’ and ‘short cells’ on the basis of their length. Short cells may contain special compounds: silica cells are of silicified (SiO2) walls or cork cells with water proof, suberinized cell walls. Bulliform cells are specialized epidermal cells of grasses living in dry, hot habitats (e.g. Festuca, Stipa, Zea). These cells occur in small groups on the leaves. The outer periclinal wall is considerably thinner than that of the other surfaces. In case of permanent drought the plant body looses water and thus the water content of the bulliform cells also declines. Due to the decreasing turgor pressure the cells become flaccid, yet owing to the uneven cell wall the outer surface becomes more constricted than the others. Since several groups of bulliform cells occur on the leaf surface, the leave consequently folds. The advantage of this process is the formation of a leaf cylinder, within which the air becomes quickly saturated with water vapour reducing the transpiration activity of the plant and thus enhancing its adaptation to the dry environment.
Some plants have water accumulating epidermis. Similarly to those of the water storage parenchyma (see later) these cells have a large, mucilage-filled vacuole. The polysaccharide (or glycopolisaccharide) content of the vacuole has a large water binding capacity. Such an epidermis can be observed in several species of Commelinaceae (e.g. Zebrina pendula).
Anatomical features of the epidermal cells may serve the adaptation to the light conditions, as well. The lower (abaxial) leaf epidermis of shade-tolerant plants is often purple due to anthocyans accumulated in vacuoles (e.g. Zebrina pendula, Cyclamen purpurascens). The purple pigment reflects the light rays that pass through the mesophyll without being utilised by the chloroplasts. The reflected radiation has a ‘second chance’ to take part in the photosynthesis.
2.4.3. Secondary dermal tissues: the periderm and the rhytidome
If the growth of a plant organ arises following the establishment of the primary tissues, we speak about secondary growth. Since usually no cell division occur in the primary dermal tissues (epidermis, rhizodermis), they cannot expand and thus they come off the surface. Of course, secondarily thickened organs also need protection, so within their outer tissue layers a secondary meristem is formed that produces a secondary dermal tissue, the periderm.
When the epidermis of a thickening organ tears off, the underlying ground tissue comes out to the surface. Some of its cells regain their dividing ability (dedifferentiate) and turn into a secondary meristem, the cork cambium (phellogen). (In the root, not the outermost cell layers but the outer cell layer of the stele, the pericycle, constitutes the phellogen, together with the neighbouring parenchyma cells.) Cork cambial cells produce new cells either toward the organ surface (monopleuric phellogen) or toward both the outside and the interior of the organ ( bipleuric phellogen). The tissue layers facing the outer world constitute the cork (phellem), while those lying on the inner side of the phellogen (which are sometimes absent) comprise the phelloderm. Therefore, the periderm is a complex tissue, built up by the phellem, the phellogen and the phelloderm.
The cells of both the phellem (‘cork’) and the phelloderm have suberised secondary walls. Due to the deposition of suberin, water cannot penetrate these cell layers and the cells themselves die. However, the periderm not only inhibits entering water into the plant organs , but also the isolates inner tissues regions from the air. So that to solve this problem and enable gas exchange, at certain spots (basically at the place of the former stomata), crater-like openings, the lenticels establish (Figure 5). In the area of lenticels, the cork ruptures and elevates from the lower cell layers. The space between the phellogen and the elevated phellem is filled with a special ground tissue produced also by the phellogen, which has numerous intercellular spaces. Through this so-called filling tissue, the air can penetrate freely into the organ. (Thus lenticels are mere periderm pullulations, established by the locally active phellogen, where intercellular spaces open between the parenchyma cells due to the accelerated cell divisions.)
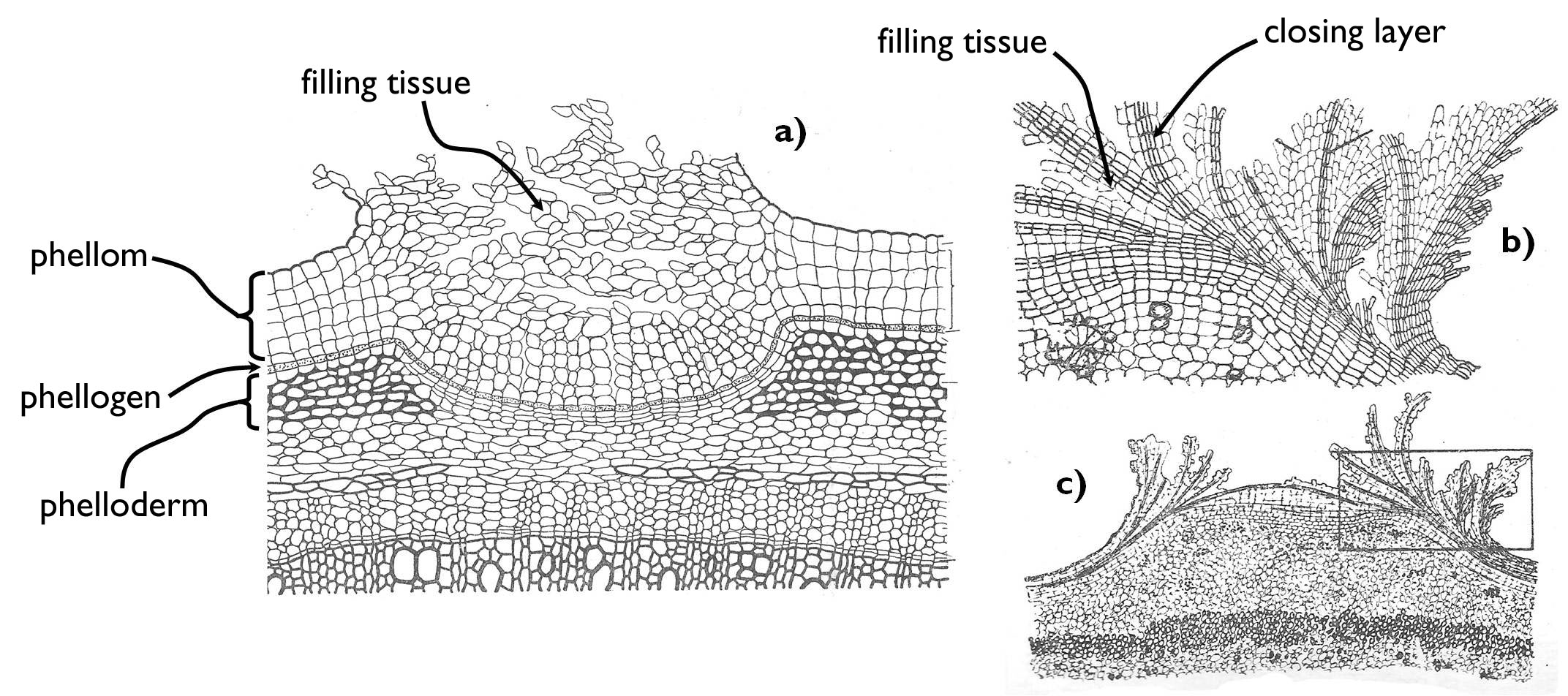
Figure 5. The structure of periderm and the lenticells.
a) Sambucus type lenticell, b-c) Gleditsia type lenticell.
(Based on Sárkány and Szalai)
The stem of the woody plants thickens year by year, so their periderm continuously expands and cracks. Meanwhile, the phellogen continuously penetrates into the ground tissue of the cortex (i.e. every year the outermost ground tissue cells turn into a meristem). However, this results the unceasing slimming of the parenchyma around the vascular tissues (i.e. the primary cortex). When there is no ground tissue left outside the phloem, the outermost phloem parenchyma cells dedifferentiate to establish the phellogen. The periderm produced by this special cork cambium thus contains cribral elements (the remnants of sieve cells, sieve tubes or cribral fibers). This phloem-derived protective tissue is the rhytidome (bark). The macroscopic features and morphology of the bark are characteristic of the species. Its colour, texture and separation properties are of taxonomical importance when identifying a woody plant.
2.5. The vascular (conducting) tissue system
2.5.1. Transport routes within the plant body
Transported compounds moves via two different pathways within the plant body (Figure 6). The first possibility is the apoplastic transport, when the materials are displaced within the aqueous solution outside the protoplasm (i.e. the cell membrane). The apoplast consists of the cell wall cavities and the intercellular spaces. The other route of transport is the symplast, i.e. the cells’ protoplasm interconnected by the plasmodesmata. Actually the term ‘symplast’ refers to all the spaces bordered by the plasmalemmae. Owing to the presence of plasmodesmata between the cells (see in Chapter 1), the whole plant body is composed of some large symplastic units.
Transported compound can move from the apoplast to the symplast through the cell membrane, either by diffusion (in case of hydrophobic substances) or protein mediated transport mechanism (for details see Chapter 1). The main difference between the displacement through the two pathways is the fact that symplastic transport can be regulated by the living cells themselves. Thus, it is of great importance if the route of a transported compound is restricted to the symplast (like in the layer of endodermis within the root).
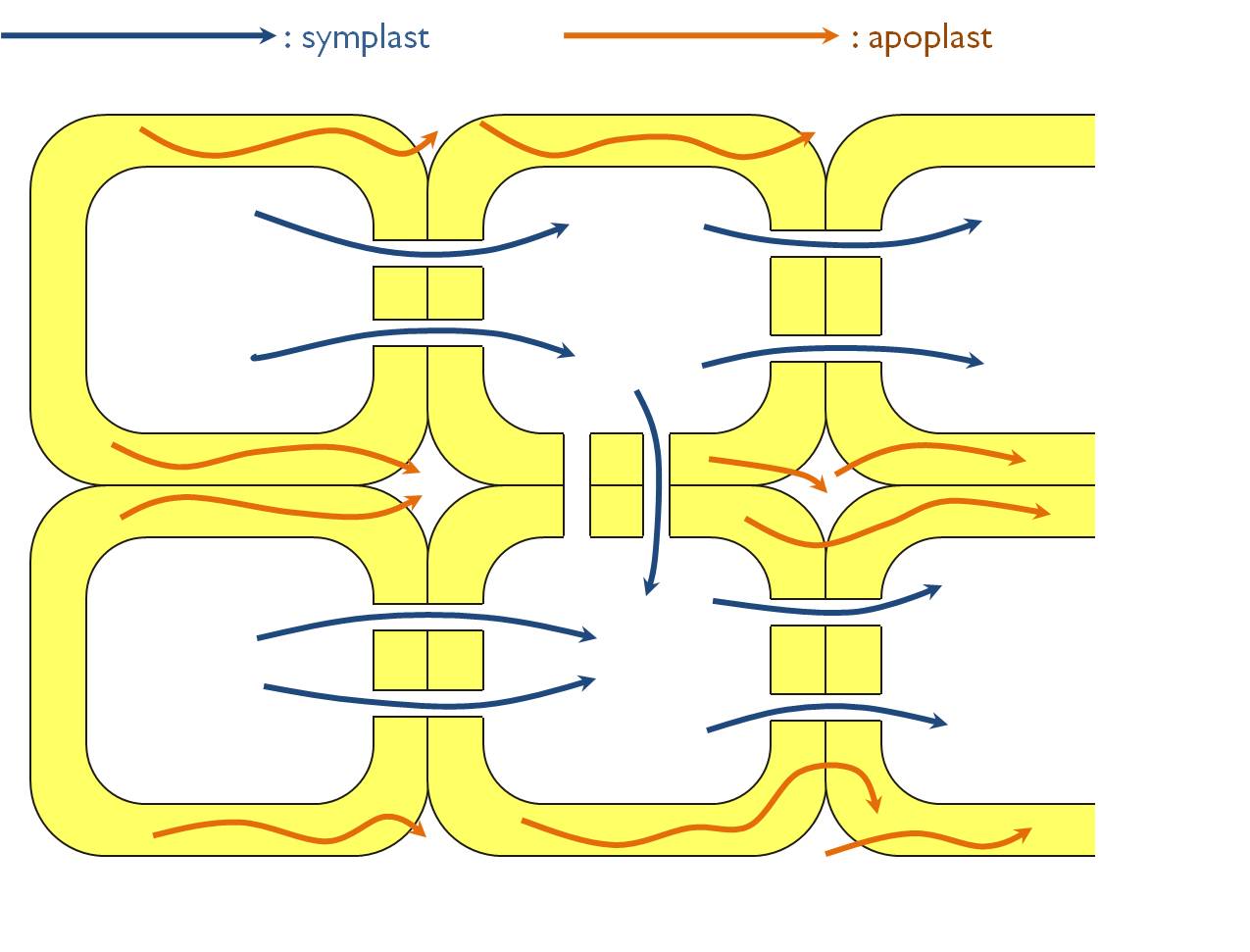
Figure 6. Possible pathways of transport within the plant body
Two main types of substances are transported within the plant body. Nutrients (i.e. water and minerals) flow from the root system toward other organs (foliage, green stem, ripening fruit etc.). Organic compounds are always transported from the place of their production (‘source tissues’) toward the tissues that utilise them (‘sink tissues’). Photosynthetic assimilates are transported as sucrose from the mature leaves to the growing parts and the storage organs. The same is the direction of displacement of amino acids. Plant hormones move from their sources toward their target tissues.
The main pathway of the polarized transport within the plant body is via the conductive tissue elements comprising the vascular bundles. However, short-distance transport between nearby cells also occurs, this is accomplished by specialised parenchymatic cells (e.g. in rays or via transfer cells – see later in details). Short-distance transport does not require specially elaborated anatomical adaptations, it is provided either by the appropriate arrangement of the cells (e.g. in the rays) or by the extended cell surface resulting stronger cell-to-cell connections (in case of the transfer cells) between the cells. To the contrary, for the purpose of efficient transport, conductive elements are elongated, they have an increased diameter (i.e. become tube-like), their protoplast chiefly degenerate so as not to hinder the transport. The elongated cells are typically strengthened by the spirally or annularly thickened secondary cell walls. Cells of the vascular tissues do not contain any chloroplasts, and low is the proportion of intercellular spaces between them.
Cells of the conductive tissue are often in intimate contact with those of the ground tissue system: vascular bundles always contain parenchymatic cells (xylem and phloem parenchyma cells) as well as supportive fibres (xylary and extraxylary fibers).
2.5.2. Composition of the vascular tissues system
The elements of the vascular tissue system can be divided into two main categories, which also spatially separate from each other. The xylem is responsible for the transport of the plant nutrients (water and minerals) from the root toward the foliage, while organic compounds (sugars, amino acids and some hormones) move from their source organs toward the utilising ones within the phloem.
Based on their ontogenetic stages we distinguish two categories among both the xylem and the phloem elements. Primary meristems (the procambium in the stem and the plerome of the root) produce the earliest developed vascular structures, the primary xylem and the primary phloem elements.
In organs thickening by secondary growth, differentiated tissues turn into secondary meristems. Among these, vascular cambium produces secondary vascular elements, the secondary xylem and the secondary phloem.
2.5.3. Xylem: structure and function
Xylem consists of tracheary elements (conducting water and minerals), parenchyma cells and supportive fibers (libriform or xylary fibers) (Table 1). The unity of tracheary and parenchymatic elements is called ‘hadrom’.
Transport within the xylem is always unidirectional (from the root toward the leaves) and driven by physico-chemical forces. The continuous osmotic water uptake by the root hairs exerts a power on the xylem solution (root pressure). The movement of this solution is also supported by thecapillary force within the tracheary elements of the xylem. The third component of water conduction is the negative pressure of transpiration stream, which is caused by the fact that the concentration of water molecules within the air spaces of the transpiring organs is much higher than outside the plant body, thus water vapour continuously leaves the tissues by diffusion 2. Since the mentioned forces do not need any living cells, the protoplasm of the tracheary elements degrades during the process of differentiation (i.e. the cells die by apoptosis) on account of the efficient conduction. Consequently, xylary elements (except parenchyma cells) are nonliving cells.
The xylem of all vascular plants contains tracheids (Figure 7). These elongated cells with lignified secondary walls form long rows above each other with their tapering ends overlapping. The cell wall of these overlapping regions have porous secondary wall with several pits (or bordered pits in case of gymnosperms), while the elongated side walls are strengthened by spiral or angular secondary thickening. Conduction occurs through the pit pairs of the oblique walls, thus along a helical pathway that inclines parallel with the longitudinal axis of the tissue.
In angiosperms, in addition to the tracheids also tracheas develop. These rather long structures (reaching a length of even several metre) consist of quite short cells of large diameter (vessel elements) interconnected due to the disappearance of their common cell walls. Owing to their admirable diameter, water conduction within the tracheas is much faster and more efficient as in the tracheids. Nevertheless, tracheas are more vulnerable to blocking by air bubbles (embolism). Embolism can be caused by the increased transpiration in arid environments or during the melting of the soil following the frost. Air bubbles easily gather and fuse, thus impede a larger area of the xylem than in case of the tracheids. To the contrary, air bubble in a tracheid is trapped at the tip corner of the cell which does not interfere with water conduction. This is the reason why more evergreen species exist among gymnosperm than angiosperm trees. In evergreens, water is conducted even also in winter (i.e. the frosty periods of the year), while deciduous angiosperms protect themselves against the embolism by losing their foliage and falling into dormancy. (Despite the dormancy in winter, the tracheas of the angiosperm xylem function for a far shorter time then the tracheids of the gymnosperms.)
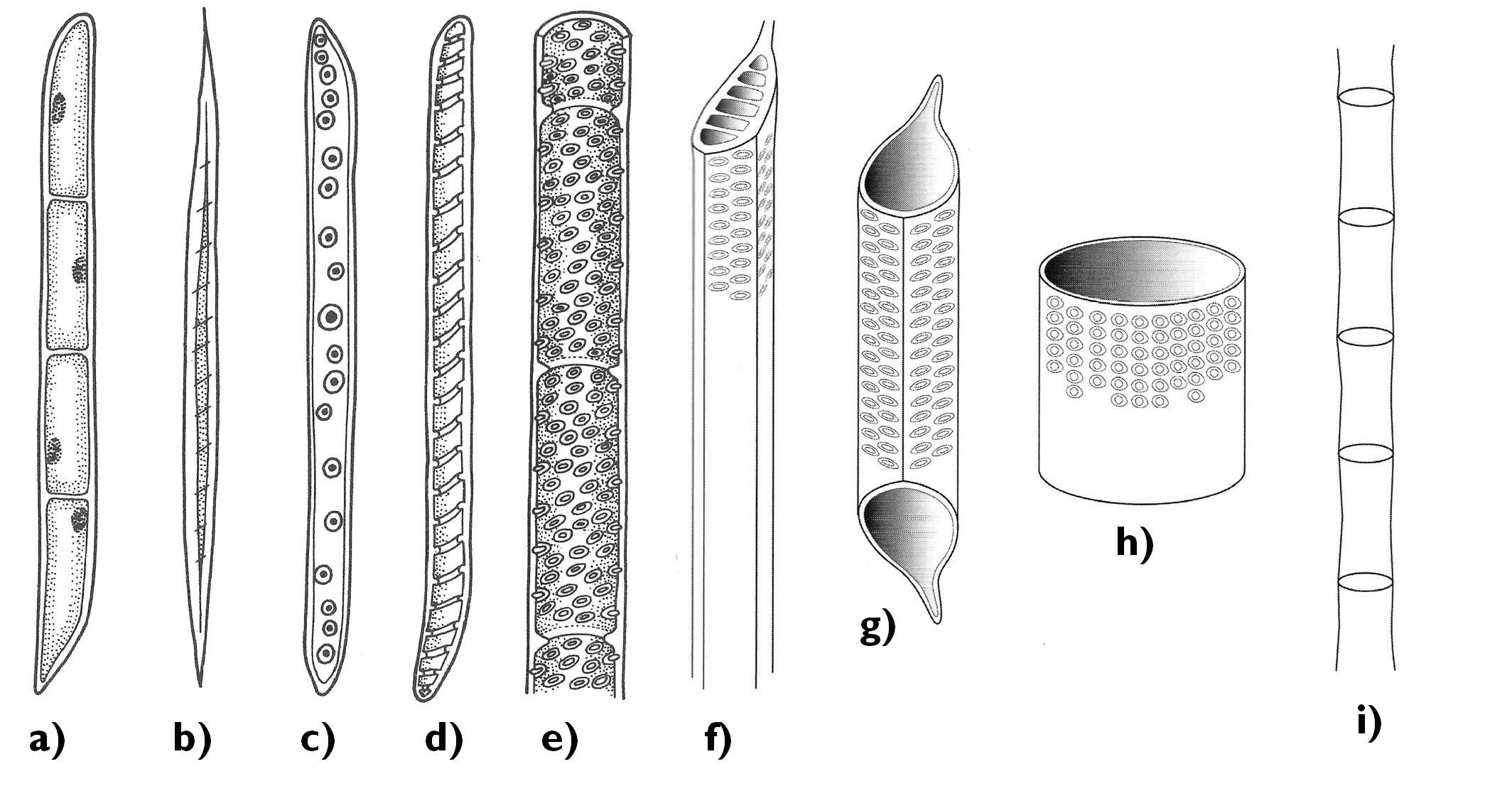
Figure 7. Elements of the xylem. a) xylem parenchyma cell; b) xylary fiber; c-f) tracheids (c: secondary wall with bordered pits, d: annular secondary cell wall thickening, e-f: pitted secondary wall); g-h) vessel elements; i) trachea.
(Based on: a-e: Jacob et al., f-i: Beck)
Beside the tracheary elements, the xylem always contains parenchymatic cells. Their function is nutrient storage and short-distance transport. Two different systems of parenchyma cells can be observed in the xylem: one arranged in parallel with the rows of tracheids and tracheas ( axial parenchyma) and the other being perpendicular to the vascular elements, comprising the rays (ray parenchyma). The function of ray parenchyma is radial conduction (often between the xylem and the phloem), as well as providing flexibility for the xylem.
The supportive components of the angiosperm xylem are the long, narrow, thick walled xylary fibers. These cells of the supportive ground tissue increase the mechanical stability of the xylem owing to their highly lignified secondary cell wall. The xylem of the gymnosperms contains no fibers but, thick walled, fiber-tracheids that serve the function of support instead of water conduction. (Gymnosperm wood contains several intermediate forms between tracheids and fiber-tracheids, from the xylem sap conducting tracheids with thickened cell walls to those with vestigial pits unable to transport the xylem sap.)
2.5.4. Phloem: structure and function
The main function of the phloem is to transport the photoassimilates (in the form of sucrose). Besides, amino acids and certain hormones are also conducted here. The conductive structures of the phloem (sieve elements) serve their function with the support of the phloem parenchyma cells (their union is called ‘leptom’). The stability of the phloem is provided by phloem fibers (extraxylary fibers) (Table 1).
Table 1. Components of the conductive tissue.
-
phloem
extraxylary fibers
leptom
phloem parenchyma cells
sieve elements
sieve cells
sieve tubes
companion cells
xylem
xylary fibers
hadrom
xylem parenchyma cells
tracheary elements
tracheids
tracheas
Organic compounds are transported from the cells of their production (‘source organs’) toward the tissues of their utilization (‘sink organs’). Consequently, in some phloem elements (e.g. in the petioles) the direction of transport may change according to the needs. Thus, transport in the phloem is a regulated process, which requires living cells with – more-or-less – complete protoplasm, so the phloem elements are living cells (except the fibers). However, the composition of the cytoplasm changes in the sieve elements: during the differentiation the tonoplast of the vacuole breaks down, the cell sap gets mixed with the cytoplasm (‘myxoplasm’) and some organelles (e.g. the nucleus) also degrade.
The smallest conducting elements of the phloem are the sieve cells (Figure 8). These elongated, tapering cells of spiral or annular cell wall thickenings pass the organic compounds to each other via the plasmodesmata extending through their common cell walls. The densely arranged plasmodesmata give a cribble-like appearance to the overlapping cell wall areas – these are the so-called sieve areas.
Beside sieve cells, the phloem of the angiosperms also contains sieve tubes. Similarly to the tracheas, the sieve tubes are also composed of short cells of considerable diameter, the sieve tube elements. The transverse common cell wall between the neighbouring sieve tube elements differentiates into a porous sieve plate. Around the pores (actually: widened plasmodesmata) callose is deposited covering later the whole common cell wall surface and finally blocking the pores of the sieve plate, what ceases the function of the sieve tube.
Metabolism and conductive function of sieve tubes and sieve cells are supported by the neighbouring cells. During the formation of the sieve tube, the sieve mother cell divides by unequal division producing the sieve tube element and another, narrower cell, the companion cell, which keeps its close connection to the tube element through several plasmodesmata. Companion cells are metabolically active cells with dense cytoplasm, exchanging different compounds with the sieve tube element. An evidence for their close interrelationship is the fact that the dying of each causes the decay of the other.3
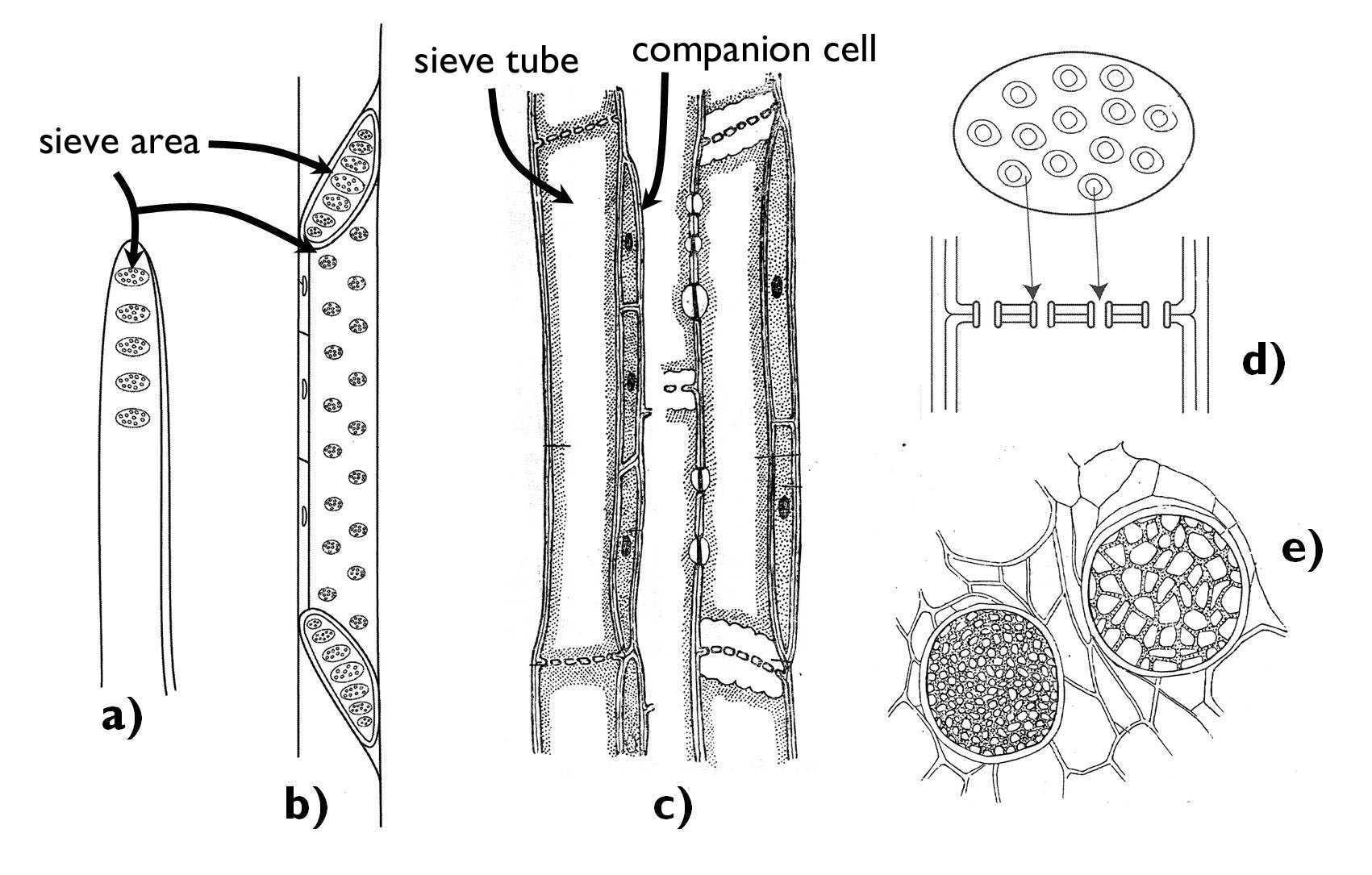
Figure 8. Different components of the phloem.
a-b) sieve cells; c) sieve tube elements with their companion cells; d-e) the structure of the sieve plate.
(Based on: a-d: Beck , e: Habelrandt)
The source organ for the sucrose transported in the phloem is chiefly the mature, photosynthesizing leaf (or else a green stem), while the sinks might be the storage organs (rhizome, tuber etc.), growing parts (young leaves, the root or the shoot tip) or the developing flowers, fruits or seeds. Accordingly, sugars move within the phloem of the petiole of young leaves from the stem toward the expanding lamina, yet following the maturation of the leaf the transport turns back and is directed toward the leaf base.
2.5.5. Vascular bundles
The elements of the conductive tissue never occur individually within the plant body, but they comprise vascular bundles. Beside the tracheary and sieve elements, bundles also contain (parenchymatic and supportive) cells of the ground tissue system.
Vascular bundles can be assorted into different categories based on their composition and the position of their components (Figure 9). Simple (radial) bundles are composed either merely of xylem or phloem elements. This type of bundle is characteristic of the primary structure of the root. To the contrary, bundles of the shoot (i.e. those of the stem, leaves and generative organs) are compound bundles comprising of both xylem and phloem. In these bundles the spatial position of the two types of vascular tissue might be various. In collateral bundles xylem and phloem run adjoining each other on an edge surface, meanwhile in concentric bundles one of them totally surrounds the other. In lamellar bundles the flat bunches of phloem alternate with those of the xylem (e.g. in the prostrate stem of Lycopodium clavatum).
If the collateral bundle of an organ is capable of secondary thickening, cambium layer can be found between the xylem and phloem. This is the collateral open bundle (e.g. in the stem of the majority of the dicots or in evergreen leaves). No cambium occurs between the xylem and the phloem of the collateral closed bundle (in the stem of the monocots or in deciduous leaves). The bundles of certain families (e.g. Apocynaceae, Cucurbitaceae) have phloem on both sides of the xylem (bicollateral bundles) 4.
Concentric bundles differ concerning the type of the central conductive tissue. In amphicribral (hadrocentric) bundles the phloem surrounds the xylem elements. This type is frequently present in the rhizomes of ferns (e.g. in Pteridium aquilinum). Phloem is in central position encompassed by the xylem in case of amphivasal (leptocentric) bundles (e.g. in the rhizome and stolons of Convallaria majalis).
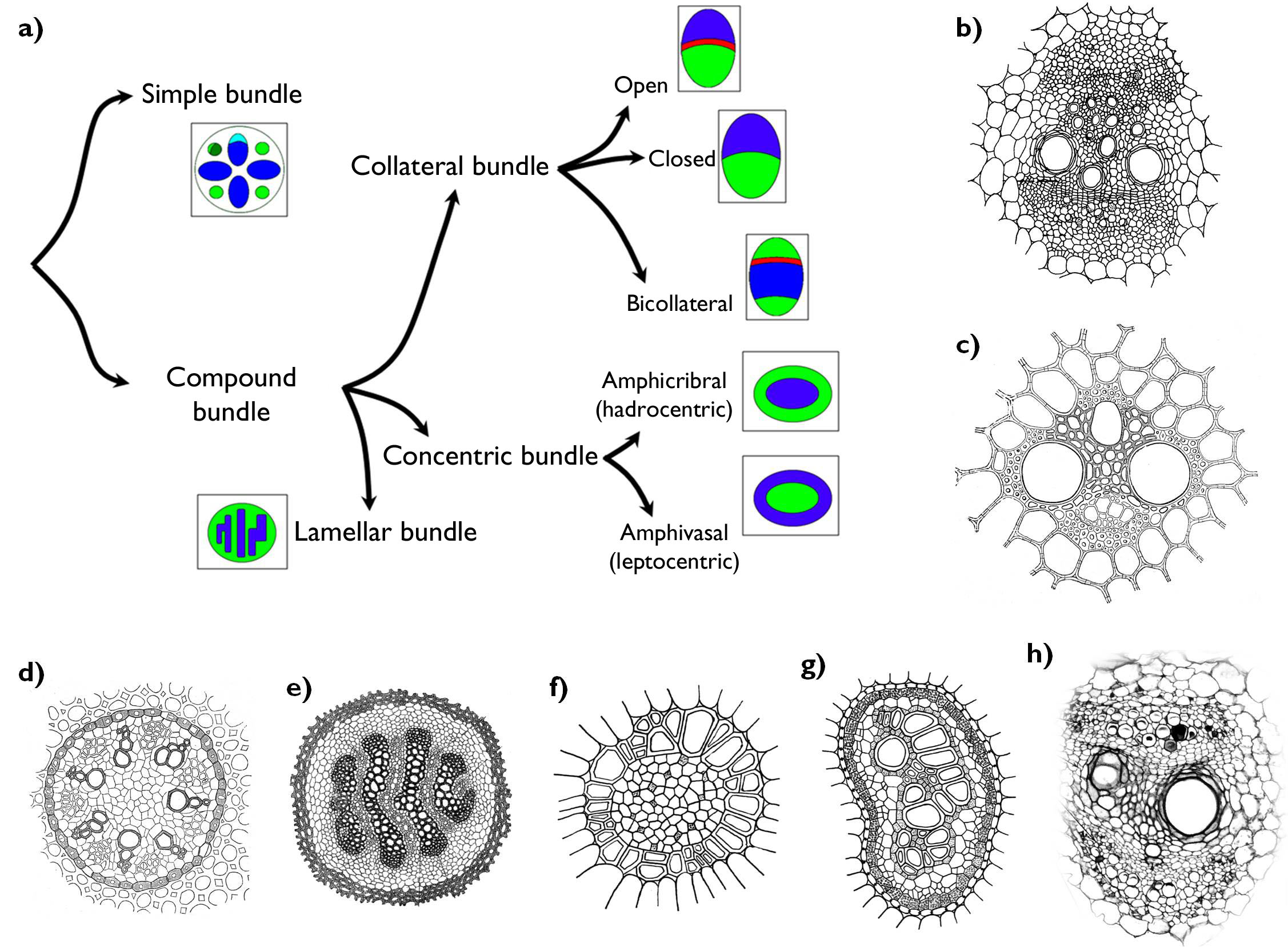
Figure 9. Different types of vascular bundles. a) the system of vascular bundles (green: phloem, blue: xylem, red: cambium); b) collateral open bundle; c) collateral closed bundle; d) simple (radial) bundles; e) lamellar bundle; f) amphivasal (leptocentric) bundle; g) amphicribral (hadrocentric) bundle; h) bicollateral bundle.
(Based on: Haraszty, c-d: Habelrandt, e: Sárkány and Szalai, f-g: Haraszty, h: Evert and Eichhorn)
2.6. Ground tissue system
The most universal differentiated tissue system of the plant body is the ground tissue system that comprises the ground substance of the organism. Cells of the ground tissues may serve various functions, thus they have no general histological characteristics. Their features vary according to the role they play in the plant body. However, this is the only tissue type that has extended intercellular spaces within. This differentiated tissue is most often predisposed to regain its dividing activity, i.e. to turn into a secondary meristem.
2.6.1. Types of the ground tissues
The most ordinary type of ground tissues is the parenchyma (Figure 10). Parenchyma cells are isodiametric, highly vacuolated and have thin secondary cell walls. They chiefly lack plastids (occasionally contain proplastids), the area of intercellular spaces is usually restricted. It is present as the pith tissue in the stem and the root or in the mesophyll of non-photosynthesizing leaves 5.
Thin-walled cells rich in chloroplasts comprise the chlorenchyma (assimilating ground tissue). It can be found in green organs, e.g. in the mesophyll of leaves, cortex of young stems or in the pericarp of unripe fruits. Its cells are of varied shapes: palisade chlorenchyma consists of densely packed, columnar cells. Among the cells of the spongy chlorenchyma extended schizogenous intercellular spaces open.
Storage parenchyma consists of cells with numerous leucoplasts accumulating different compounds. It is found in different storage organs (in the cortex of the storage tap root, rhizomes or tubers). One of its sub-types is water storage parenchyma characteristic of succulent plants. Its cells have a large vacuole filled with hygroscopic polysaccharide (mucilage) that absorbs high amount of water. Aerenchyma has large, air-filled intercellular cavities, which allow ventillation of the organs submersed in water or growing in a soil with high water content.
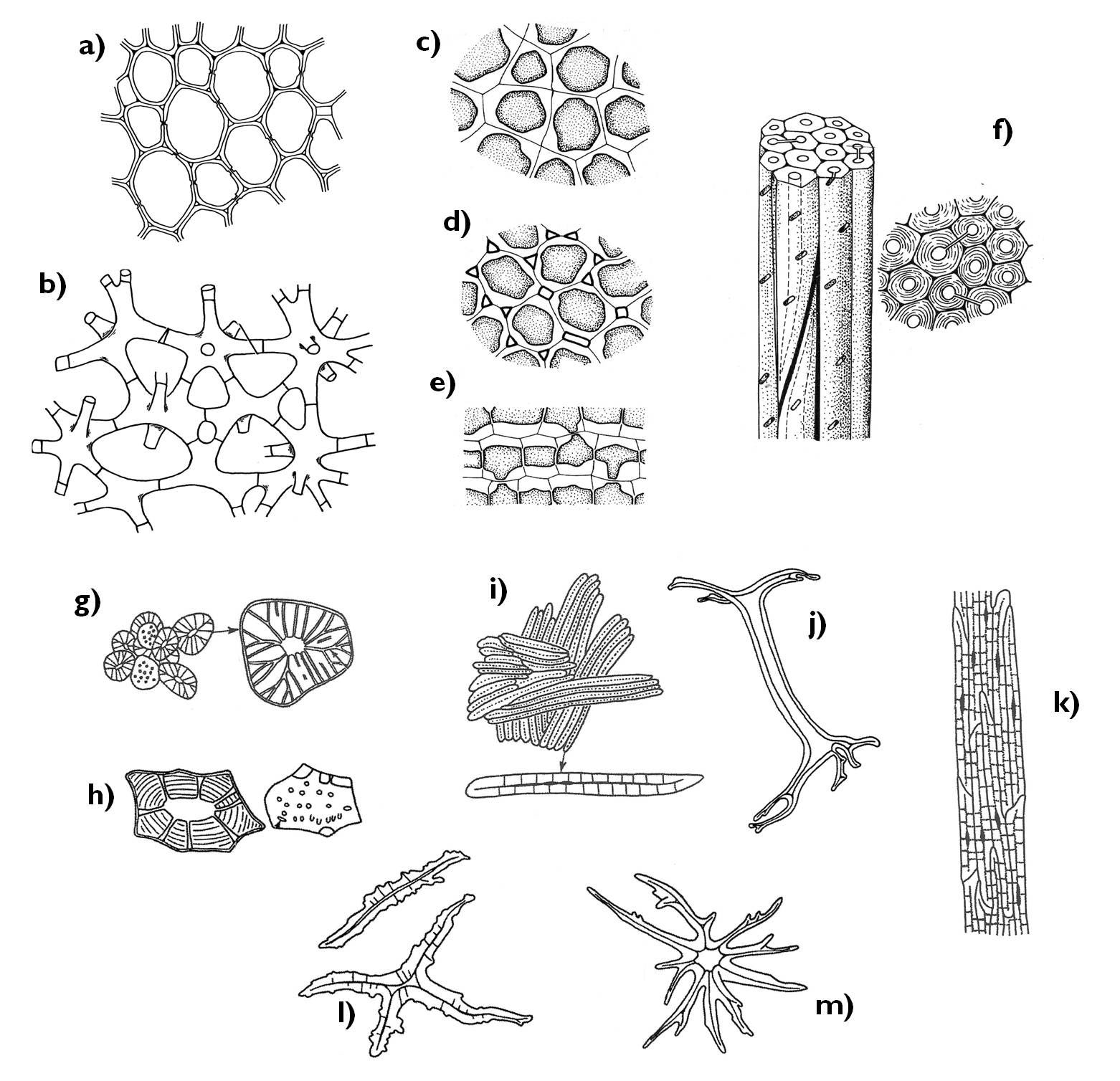
Figure 10. Different types of the ground tissue. a) parenchyma; b) aerenchyma; c-e) collenchyma (c: annular collenchyma, d: lacunar collenchyma, e: lamellar collenchyma); f) sclerenchymatic fibers; g-m) sclereids (g-h: brachisclereids (Pyrus, Hoya), i-k: macrosclereids (Malus, Hakea, Allium), l-m: asterosclereids (Camellia, Trochodendron)).
(Based on: a-b: Evert and Eichhorn, c-f: Jacob et al., g-m: Beck)
The function of supportive ground tissues is to provide rigidity and stiffness for the plant organs, as well as the protection of the more delicate inner structures. All of their cells possess quite thick secondary wall, yet the composition of the cell wall may be different. In case of collenchyma, layers of cellulose, hemicellulose and pectin are deposited onto the primary wall. This type of supportive tissue usually forms hypodermal layers (i.e. it is right under the epidermis). It supports the cortex of young stems, the leaves in the vicinity of the leaf veins, the pedicel of flowers and fruits, but it also occurs in the pericarp.
The other type of supportive ground tissue is sclerenchyma. Its elements have intensely lignified cell wall, which isolates the cells so much from their environment that makes them die. There are two forms of sclerenchyma in plants: sclereids and fibers. Sclereids are thick-walled cells of various shapes usually scattered within other tissues. Star shaped asterosclereids stiffen the aerenchyma of water-plants. Small groups of isodiametric brachisclereids (stone cells) can be found dispersed in the flesh of the Pyrus fruits, yet the same cell type comprise the hard endocarp (‘stone’) of drupes, and they are also present in the cortex of certain stems (e.g. that of the Hoya species). The seed coat of some species contain columnar macrosclereids or bone shaped osteosclereids (e.g. in Phaseolus species).
Narrow, considerably thick-walled elongated supportive elements (often reaching a length of several centimetres) are the fibers. Two main types are the xylary fibers associated with the xylem, and extraxylary (phloem) fibers attached to the phloem. Formers have more intensely lignified, thicker walls and derive from common meristematic mother cells of other xylem elements. They might be either long, elongated, libriform fibers or fiber tracheids of quite thick cell walls. These elements represent a transitional stage between tracheids and libriform fibers. Fiber tracheids are the sole supportive cells of the xylem of gymnosperms. Extraxylary fibers have characteristically thinner cell walls than the xylary ones, but their length usually exceeds considerably the length of the latter (e.g. phloem fibers of Boehmeria nivea may reach 55 cm). Almost all fibers are attached to vascular bundles, either as an integrated element or surrounding the bundles forming an open or closed bundle sheath. Bunches of extraxylary fibers often run adjacent to the phloem forming a “bundle cap” or “phloem cap”.
It is worth mentioning that the term ‘fiber’ used by the industry is not necessarily the same as used by plant anatomy. Mostly, ‘industrial fiber s’ are complete vascular bundles together with their surrounding bundle sheath tissues. Phloem fibers of the hemp (Cannabis sativa) are used for producing rope, string, thread or coarse canvas subsequent to removing the xylem by soaking and scutching. Flax fibers (Linum usitatissimum) consist almost completely from cellulose, used for producing fine linen. Likewise, the seed coat hairs of cotton (Gossypium) are almost entirely clear cellulose. These epidermal hairs are industrial fibers, too.
2.6.2. Secretory ground tissue and inner secretory structures of plants
In addition to the modified epidermal layers and outer secretory structures, cells capable of secretion occur also within the plant organs. These cells of ground tissue origin are either scattered in other tissues (secretory idioblasts) or form sac- or vesicle-like cavities or tubular secretory canals. Unlike idioblasts, in secretory cavities (sacs) and secretory canals (tubes), the metabolic product accumulates in the intercellular space.
Complex inner secretory structures are the resin ducts, generally present throughout the wood and ground tissues of most gymnosperms, as well as in some angiosperm families (e.g. Anacardiaceae). The pericarp of hesperidia of the family Rutaceae contains oil cavities (Figure 11). The products of these secretory structures protect the plant against insect chewing due to its bitter or hot taste.
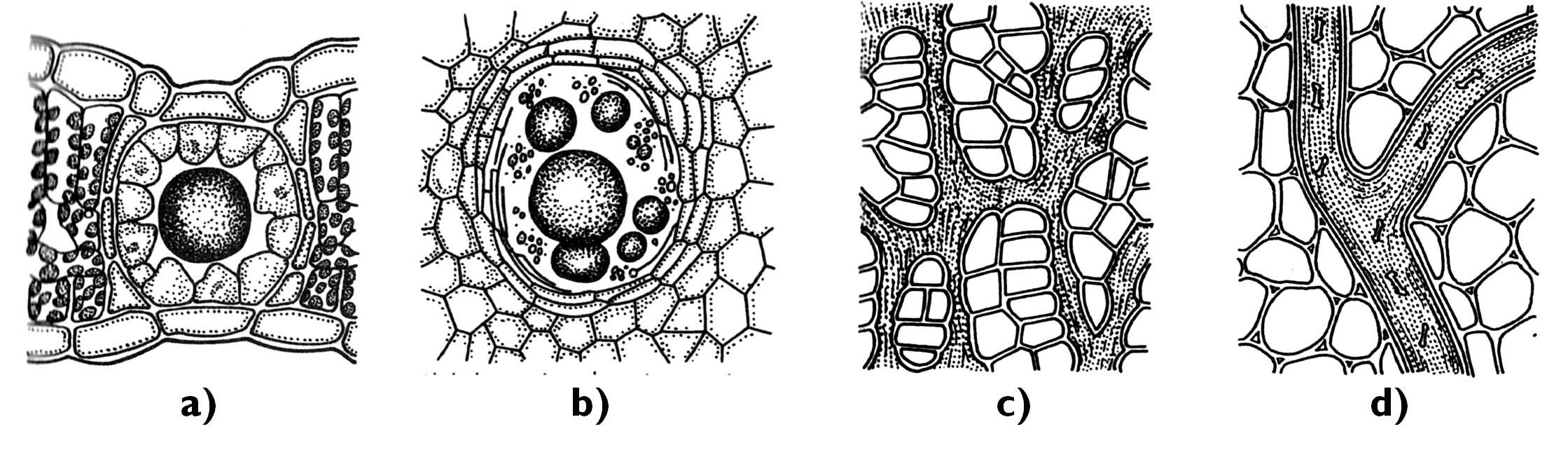
Figure 11. Internal secretory structures. a) Schisogenous oil cavity (Hypericum);
b) lisigenous oil cavity (Citrus); c) articulated laticifer (Scorzonera); d) non-articulated laticifer (Euphorbia)
(Based on Jacob et al.)
Tubular internal secretory structures are the laticifers (milk ducts) comprising an inner labyrinth-like system permeating various organs of the plant. Concerning their origin, they are either simple laticifers consisting of a sole elongated cell or compound laticifers deriving as union of several cells.
The mixture of secreted materials accumulated in the laticifer is called latex 6, which is either an aqueous solution or a colloidal emulsion of different compounds (among others lipids). It might be a clear, watery fluid (Morus, Humulus, Nerium), white and milky ( Asclepias, Euphorbia, Ficus, Lactuca) or colourful (Cannabis, Papaveraceae). Most common components of latex are saccharides (sugars, starch grains), organic acids, salts, sterols, fats, terpenoids (e.g. rubber), alkaloids and proteins (even enzymes – e.g. digestive enzymes in the latex of Carica papaya). The latex of several species is a basic material for industry (e.g. the rubber that is the latex of Hevea brasiliensis) or the source of active agents for pharmacy (e.g. opiates are derived basically from the latex of Papaver somniferum called opium, while the latex from the species of the family Apocynaceae is the major source of vinca-alkaloids). Besides, due to the high concentration of alkaloids in their latex, several of our ornamental plants are toxic for humans (e.g. Nerium oleander, Vinca minor).
2.7. Emergences: organ appendages formed by different tissues
Emergence s (appendages) represent an intermediary level of organization between that of tissues and organs. In addition to the epidermis, they are composed of other sub-epidermal tissues (ground tissue, rarely vascular elements), thus they cannot be assigned unambiguously to any tissue system, yet they are no independent organs either.
Emergences are for instance the prickles of Rubus and Rosa species or the cushion-like protuberances on the shoot surface of the genus Urtica, composing of ground and epidermal tissues and bearing the stinging hairs. Similarly, insect trapping, glutinous tentacles of the sundew (Drosera) belong to this category, too. The head of this stalked structure is covered by secretory epidermis producing sticky mucilage and digestive enzymes, and in the parenchyma of its interior conductive elements (tracheids, sieve cells) are embedded. Conductive structures of the tentacles are responsible for the transport of the precursor for the secreted products, as well as the displacement of absorbed end products of the digestion.
Refereneces:
Beck CB (2010): An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century. 2. kiad. Cambridge University Press, Cambridge,
Biebl R, Germ H (1967): Praktikum der Pflanzenanatomie. 2. kiad., Springer-Verlag, Wien-New York.
Braune W, Leman A, Taubert H (1967): Pflanzenanatomisches Praktikum. VEB Gustav Fischer Verlag, Jena.
Cutler DF, Botha T, Stevenson DW(2008): Plant Anatomy: An Applied Approach. Wiley-Blackwell, Oxford.
Evert RF, Eichhorn SE (szerk.) (2006): Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body – Their Structure, Function, and Development, 3. kiad., Wiley-Interscience, New Jersey.
Gyurján I (1996): Növényszervezettan. Egyetemi jegyzet. ELTE Eötvös K., Bp.
Haberlandt G (1924): Physiologische Pflanzenanatomie. Verlag von Wilhelm Engelmann, Leipzig.
Haraszty Á (1998): Növényszervezettan és növényélettan. 6. kiad., Nemzeti Tankvk., Bp.
Jacob F, Jäger EI, Ohmann E (1985): Botanikai kompendium. Natura, Bp.
Kárpáti Z, Görgényi L, Terpó A (1968): Kertészeti növénytan I. Növényszervezettan. Mezőgazdasági Kiadó, Bp.
Mauseth JD (2008): Plant Anatomy. The Blackburn Press, New Jersey.
Sárkány S, Haraszty Á (1995): Növényszervezettan biológia-kémia, biológia ill. pszichológia-biológia szakos hallgatók részére. Egységes jegyzet. Nemzeti Tankvk., Bp.
Sárkány S, Szalai I (1966): Növénytani praktikum I. Növényszervezettani gyakorlatok. 3. kiad., Tankvk., Bp.
1 Extrafloral nectaries may also aid the pollination, like those of the euphorbias (Euphorbiaceae).
2 If the air around the plant is saturated by water vapour, transpiration ceases. That is why the intensity of transpiration can be reduced by epidermal hairs, trapping water molecules and thus the diffusion rate decrease.
3 Albuminous cell s (Strasburger cells) have a similar interrelationship with the sieve cells in the phloem of the gymnosperms. However, the exact functioning of these cells is still to be understood.
4 On one side of the xylem neighbouring the phloem, cambium can also be observed in the bicollateral bundle.
5 Some authors distinguish three main types of ground tissues: parenchyma, supportive ground tissues and endogenous secretory tissues. In this concept chlorenchyma, storage parenchyma and aerenchyma are all modifications of the parenchyma.
6 Latex might be produced also in single secretory cells scattered in any part of the plant (e.g. in Solidago species).

