Population interactions, population collectives. Plant communities: structure, functioning and dynamics
Author: Mária Höhn
Populations that share similar habitats constitute communities in which a variety of organisms interact with each other. The nature, intensity and direction of interactions depend on the availability of resourses that individuals use in order to survive, grow and reproduce. We can perceive the interactions between populations when we posess information about species’ ecological requirements and the role each population plays in a system. First, we have to identify the environmental resources used by a population and the range of environmental factors populations are able to tolerate, i.e., the population environmental tolerance. We have seen in Chapter 6 (Figure 2) that populations have a tolerance range to each environmental factor. If we add up the requirements of a population to all environmental factors (n factors), we obtain a multi-dimensional tolerance space, termed ecological niche.
7.1. The ecological niche
- is a tolerance space defined by a species’ ecological requirements;
- is a multidimensional and abstract space defined by all environmental factors required by a species;
- is occupied when a species' population is present;
Several ecologists provided an interpretation for the ecological niche concept (e.g., Joseph Grinnel 1917, Charles Sutherland Elton 1927), but a modern ecological approach came from George E. Hutchinson (1957, 1978) (Figure 1.)
Take three environmental factors. To define the tolerance (i.e., response) of a population to these factors, we first identify the tolerance limits of the population to each environmental factor and then connect all thresholds to obtain a three-dimensional tolerance space. By addig each time a new parameter, the tolerance space enlarges with the fourth-, fifth, etc. axes, producing a four-, five, etc. dimensional space or a ”hypervolume” (Figure 2.).
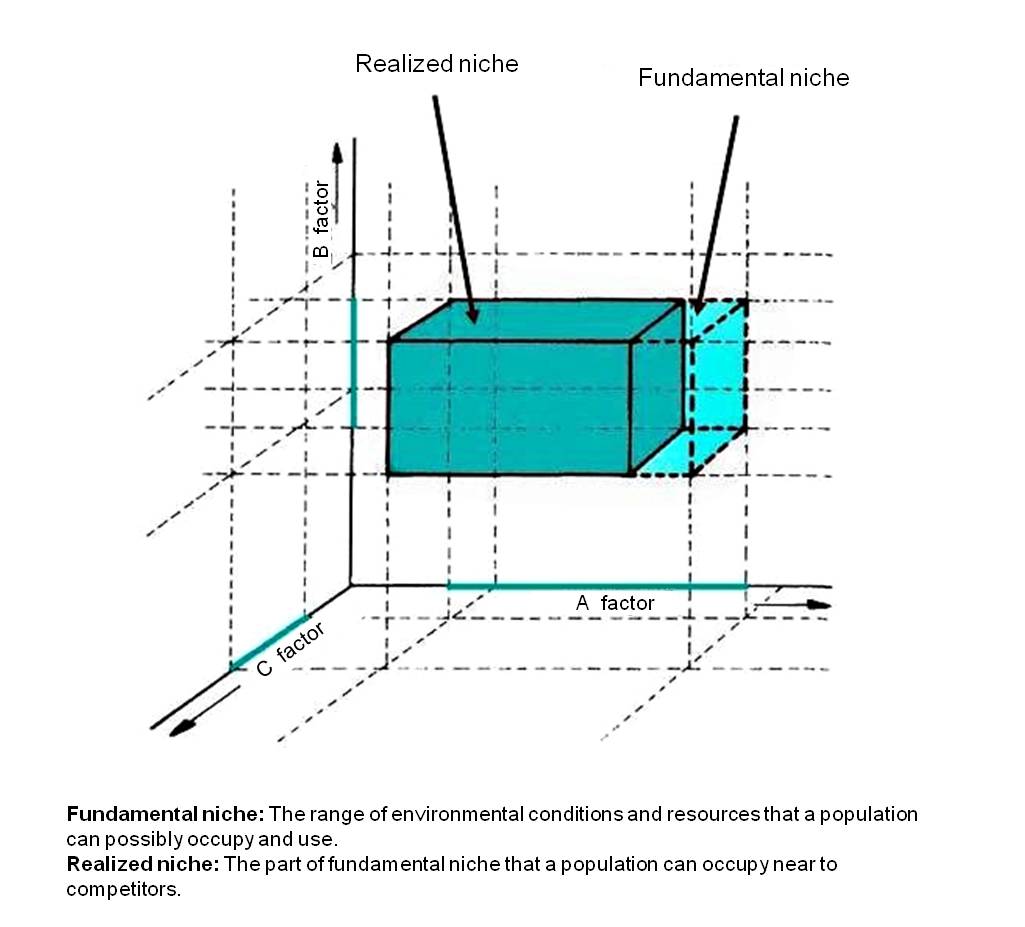
Figure 1: Representation of Hutchinson’s ecological niche: a three-dimensional tolerance space (Turcsányi (1998))
The fundamental niche represents an ecological space defined by the full range of favorable environmental conditions a species is theoretically capable of using. The realized niche is a subset of the fundamental niche, occupied in the presence of limiting factors (e.g., competitors). It is very often the case that the same environmental factors of a habitat are suitable to and used by populations of several different species. It results that the realized niche is most often smaller than, or at most equal to the fundamental niche.
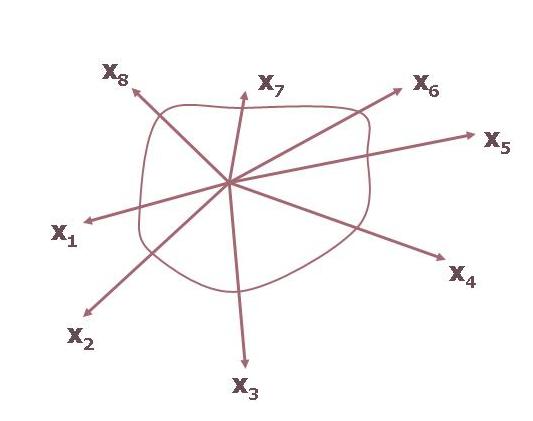
Figure 2. A representation of the ecological niche. Each axis represents an environmental factor.
Making an an analogy, Miller (1927) defined the niche as a species’ profession in an ecosystem, and the habitat as its address.
The ecological niche may be used to compare populations. If the niches of two populations overlap either partially or completely, that means the range of environmental conditions they use is very similar (Figure 3). When niches overlap, competition is highly likely to occur.
Several types of niche overlap exist:
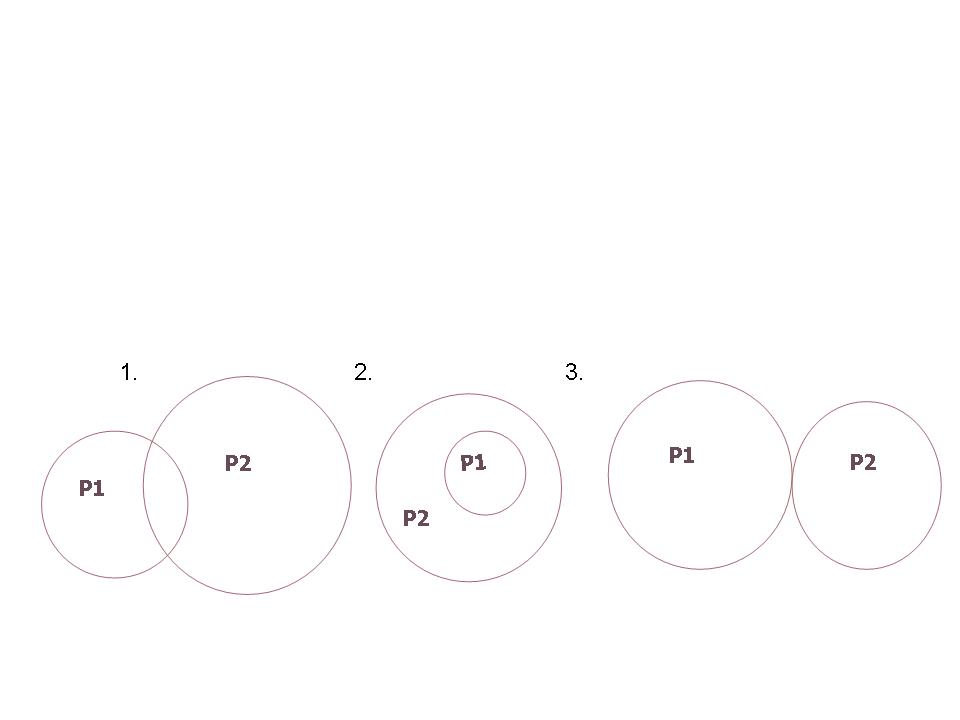
Figure 3. Different types of overlapping niches.
1. Reciprocally overlapping 2. Included 3. Abutting (may be indicative of past niche overlap)
According to Gause’s competitive exclusion hypothesis, completely overlapping ecological requirements (i.e., niches) inevitably trigger competition between two populations, one of which is usually excluded from the system.
If the niche overlap is incomplete, or niches segregate as a result of competition, two populations may persist in the same area, i.e. they coexist.
Consequently, only populations whose niches are abutting or at least are aligned along an environmental gradient will be able to coexist in a habitat, and ultimately to build up plant communities.
A niche gradient can be represented as species’ ecological optimum curves along each niche dimension (Figure 4).
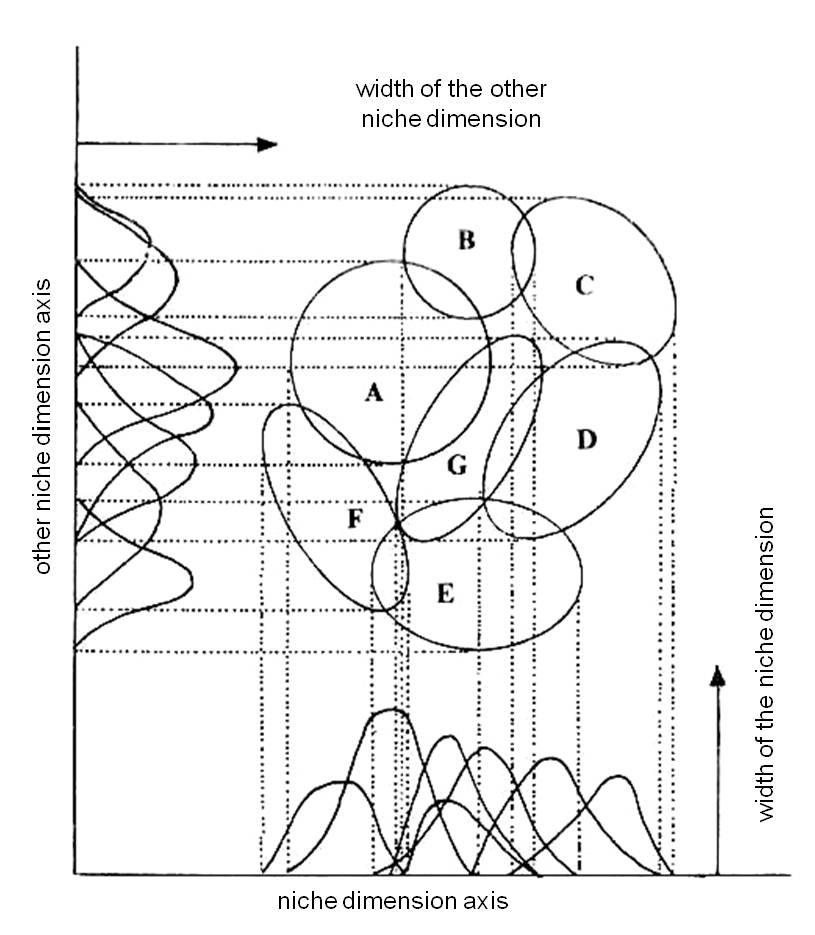
Figure 4. A niche gradient (the extent of niche overlap and alignment along a resource gradient) (From Széky Pál, ...)
7.2. Biological interactions
The niche concept helps us understand the relationship between different types of organisms. Some species don’t develop detectable relationships, because their niches are theoretically distinct. This is called neutralism. When a relationships does develop, it can take many forms (Figure 5):
- detrimental relationships, which may be
two-sided (reciprocally detrimental), e.g., competition
one-sided (detrimental to only one of the interacting organisms), e.g., parasitism and allelopathy
- beneficial relationships, e.g., symbiosis, mycorrhiza and mutualism
- loose relationships (beneficial to one, and neither beneficial nor detrimental to the other organism), e.g., commensalism
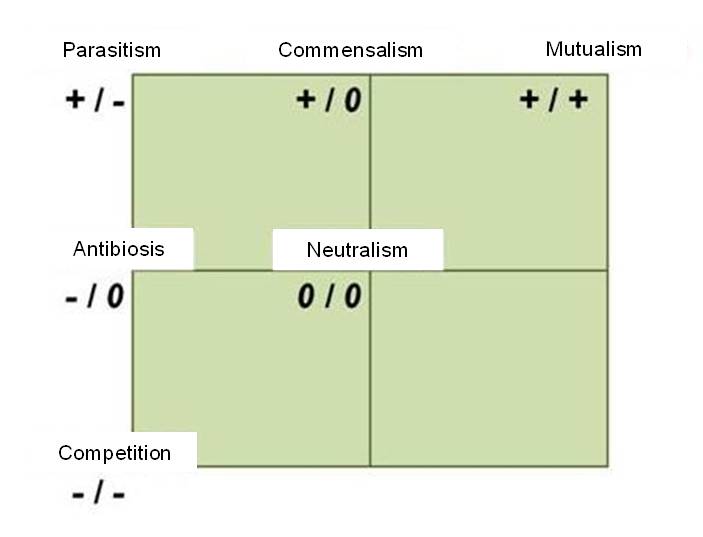
Figure 5. Different types of biological interactions and their expected outcomes
7.2.1. Competition
Competition is the most frequent of all biological interactions, which dramatically affects populations in a habitat and usually lowers significantly the number of individuals. Organisms will compete for limiting environmental factors only, which will be accessed by the most successful competitors. A resource is an environmental factor essential for growth, reproduction and survival of organisms, and therefore competition occurs for resources (see overlapping niches).
Some resources are particularly important to living beings. For example, light, water and soil nutrients represent resources for autotrophic plants. If one of these factors reaches the tolerance limits of a population, it will act as a limiting environmental factor (i.e.,limiting resource). At critical thresholds, populations may go extinct. Not all resources are limiting. For example, the atmospheric CO 2 is essential in the process of photosynthesis, but it is largely available at a constant rate to all plant individuals.
Liebig’s law of the minimum refers to the accessibility of soil nutrients, and states that growth is controlled by the limiting environmental factor, and not by the quantity of other available resources.
The outcome of competition
- Competitive exclusion (one of the competing populations is eliminated from the system)
- Coexistence (both populations continue to utilize resources in the same habitat, occupying and adjusting their realized niches)
The „niche adjustment” depends on the degree of plasticity organisms are capable of, which is in turn related to their niche breadth.
Thus in exploitation competition (or exploitative competition), the efficiency of resource use is critical. Organisms that use more of the resources will grow and reproduce faster, and will eventually outcompete others. On the contrary, organisms deprived of resources will have lower chances to grow and reproduce, and as a result their resource use rate will decrease even more with time.
The belowground root competition is a clean-cut example of exploitation competition, because competitors with longer and/or more extensive root systems are likely to utilize more successfully the soil resources.
In order to be successful, organisms have developed competitive strategies, (which can be demonstrated experimentally).
In communities of herbaceous perennials, interspecific competition produced two types of very frequent competitive strategies, first described by Schmid and Harper (1985). These are the phalanx and guerilla strategies
Plants with thick rosettes employ the phalanx strategy. They spread as a front, covering the soil relatively quickly and preventing the establishment of other colonizers. The English daisy (Bellis perennis) is a typical phalanx plant, but several other dicotiledonous species such as Hieracium pilosella or Plantago lanceolata have a similar strategy.
Plants with long internodes employ the guerilla strategy. Their horizontal stems (stolons, rhizomes) grow fast and are able to escape the local scenes of competition, while exploring suitable patches further apart. A tipical guerilla plant is the Self-heal (Prunella vulgaris), but the strategy of Fragaria species (e.g., the strawberry) is very similar.
Question rises, which strategy is more successful? It always depends on the initial situation: if density of individuals is high, guerilla species are fast in finding unoccupied patches and competitively advantageous situations; if vegetation density is low, phalanx species are better at exploiting locally the available space and resources.
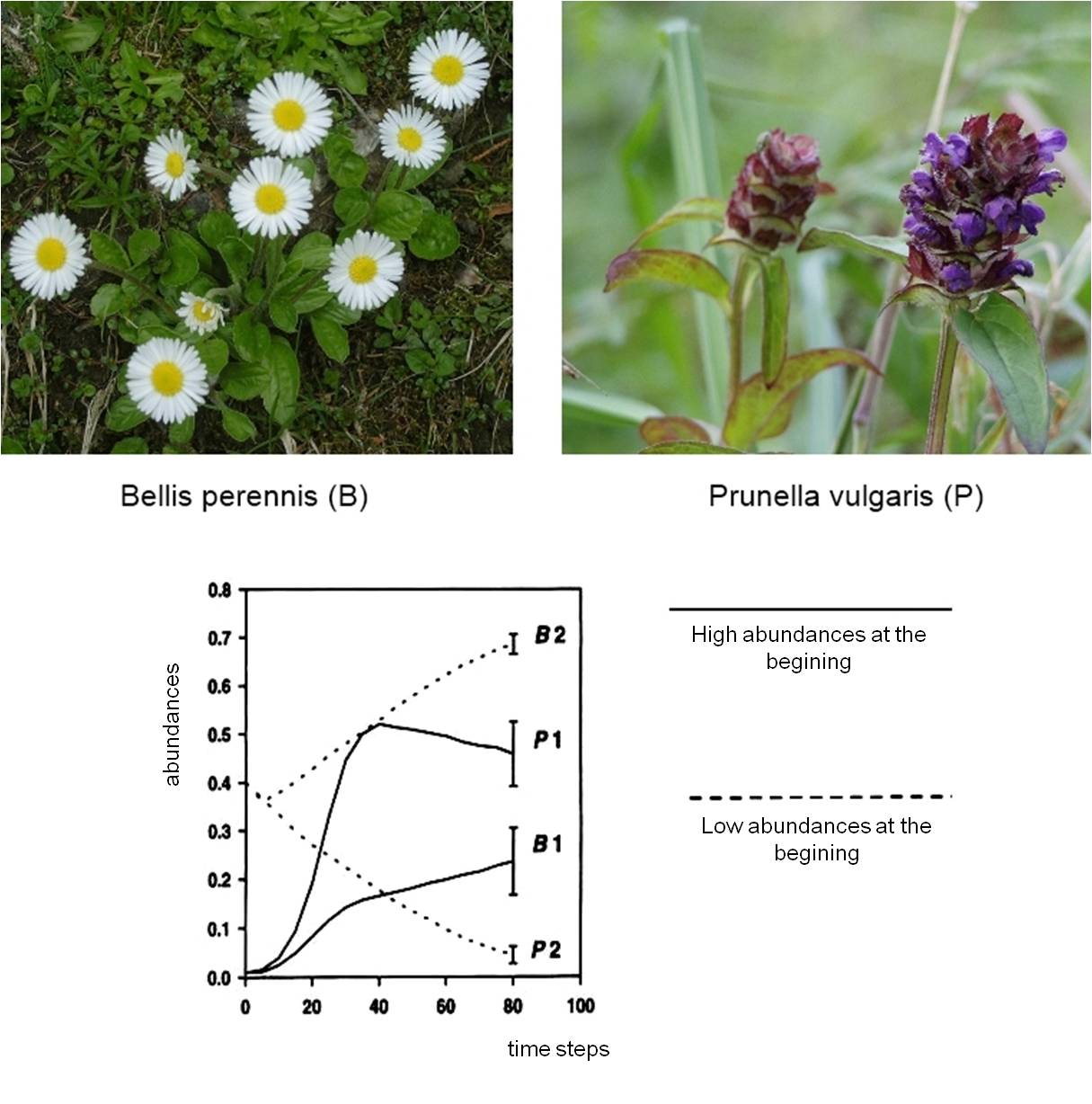
Figure 6: Experimental demonstration of the phalanx and guerilla competitive strategies with two tipical plants, Bellis perennis (B) and Prunella vulgaris (P) (From Schmid and Harper 1985)
In competition, each „interested” half is mutually involved. The competition may be interspecific (between individuals of different species) and intraspecific (between individuals of the same species). We have seen above an example of interspefic competition.
In the case of intraspecific competition, individuals of the same species compete with each other, and the strongest wins. For example, in plants, individuals that grow faster and bigger are likely to be more successful. Initial advantage also carries interest, as with time other resources will be accessed even more easier. Think about seedlings in a clearing understory. Individuals which are quick in developing leaves will be the ones to grow faster and will progressively shadow the siblings of the same cohort. Ultimately, the most successful competitors for light will also be able to invest more in their root system, which will considerably ease the uptake of resources.
Intraspecific competition is critical in the development of progeny. Take the example of hatchlings in a clutch. If food is scarce, the hatchlings who do better at stretching and squirming will take the food from their siblings, who will weaken and progressively loose the chances to survive, even if food becomes eventually abundant.
Competition between modules is another form of intraspecific competition in plants, which is essentially a competition „within the individual”. The modular structure (see Chapter 5) makes it possible for plants to regulate the dynamics of their modules and thus to increase the individual’s chances to survive. The competing modules behave similarly to individuals in a population. The ones that grow faster acquire more light, and the ones that root faster take up more nutrients.
Clonal species are able to regulate the number of modules as well as the life cycle of each module, and therefore may be more successful in both interspecific and intraspecific competition situations.
For example, thanks to an extensive network of belowground rhizomes, the raspberry (Rubus idaeus) is able to exploit maximally the soil resources. There is hardly any other plant species able to establish within a raspberry stand in a natural upland habitat. Axillary rhizome buds produce a large number of shoots which sooner or later cast shadow on its neighbours, affecting each other’s growth and productivity (Figure 7). The lifespan of a raspberry module in the wild is of three years. In the first year a leafy, green module develops; the second year is the year of maximal flowering and fruiting; the third year, the lignified modules develop only a few topical leaves and mostly don’t make any fruits. This functional partitioning helps the individual to minimize the intraspecific competition between different modules, while it maximizes the utilization of light and soil nutrients. Such modular dynamics increase significantly the fitness of raspberry plants and the competitive advantage they have over other species.
Raspberry growers take into account the plant’s life cycle. They remove the old modules but keep the unproductive first year modules (which are not worthless, since they are going to fruit the coming year).

Figure 7. The raspberry (Rubus idaeus) and its modular structure
Vine prunning serves the same purpose of minimizing the competition between different modules.
Consequently, the infraindividual (within individual) competition is a result of the modular structure of plants and in essence it occurs when different ramets of the same genet compete with each other.
7. 2. 2. Allelopathy
One specific type of successful competitive strategy is when one organism exerts negative impact on the other through chemical inhibition. Although the effect was long known, the first description was provided only in 1937 by Hans Molish. Some plants release certain types of chemical compounds from their below- or aboveground, live or dead body parts, which inhibit the germination and growth of other species’ individuals.
It is well known that the walnut (Juglans regia) leaves contain juglone which has strong allelopathic effects. The coumarins released by legumes (plants in the Pea Family) also strongly inhibit seed germination. In stands of the Black locust (Robinia pseudacacia) one can hardly find any herbaceous plants, and most of them would grow in the moist spring soil in which the toxic allelopathic compounds are being diluted. The Black locust leaves will exert their allelopathic effect only later in the summer (Figure 8). Tannins secreted by plants in the Fagales order are also strong germination inhibitors. It may often happen that mother plants regulate the development of a new generation of offsprings by releasing inhibiting, self-toxic chemical substances.
We often don’t know the composition of allelopathic chemical substances. However, it is certain that allelopathy provides strong competitive advantages to plants of different types of communities (e.g., weedy or dry habitat environments). Interestingly, the impact of gradually concentrating allelopathic toxins within dry habitats may be ceased by wildfires. Regular savanna wildfires for example make a great service to plants, as many accummulated allelopathic compounds are being burned.

Figure 8. Spring and summer aspect of a Black locust forest (Source: http://akacosut.blogspot.hu)
7. 2. 3. Parasitism
Parasitism is an uneven relationship between individuals of two different species, whereby the parasites find suitable living conditions while directly exploiting the host, and as a result the density of the host population changes.
Several types are known:
A. Obligate parasites - are those parasitic organisms whose life cycle is dependent on the host plant. They can be:
- Holoparasites, which are totally dependent on the host plant for all nutrients. Such are the parasitic fungi, e.g. the powdery mildew, downy mildew (Figure 9) and the grey mold.
Flowering plants also have their parasites. In some plant families all species are holoparasites, such as the dodders (Cuscuta sp.), Dutchmen's pipe (Monotropa sp., Figure 10) and the broomrapes (Orobanche sp., Figure 11). These plants completely lack chlorophyll and are unable to photosynthesize.

Figure 9. Symptoms of powdery mildew (Plasmopara viticola) on a Common Grape Vine leaf (Vitis vinifera)
Figure 10. Dutchmen's pipe (Monotropa hypopitys). Photo: Gábor Bottlik
Figure 11. Broomrape (Orobanche hederae) Photo: Sándor Barabás
- Hemiparasites posess chlorophyll and are therefore able to photosynthesize, but depend on a host to acquire the water necessary for this process. The Common Mistletoe (Viscum album) and the Yellow Mistletoe (Loranthus europaeus) are hemiparasites.

Figure 12. The Common Mistletoe (Viscum album)
B. Facultative parasites are those organisms which are primarily parasites, but are not totally dependent on a host plant and are therefore capable of saprophytic life. Many fungi are factultative parasites.
Parasites can be classified as:
- ectoparasites - live outside the host’s body, which they penetrate with various suctorial formations to extract nutrients
- endoparasites – such as various fungi and baceria, which live inside the host’s body
A distinct group of parasitic organisms are the wound parasites such as the hoof fungi (e.g., Fomes, Phellinus, Figure 13), which are only able to establish on wounds.

Figure 13. The Willow Bracket (Phellinus igniarius)
7. 2. 4. Interactions for food resources. Herbivory and its consequences
Nitrogen-poor habitats such as peatbogs and tropical soils are inhabited by many carnivorous plants. To supplement the amount of nitrogen, these plants use traps (modified plant organs) to catch insects and other small arthropodes, which are then digested with enzymes that break down proteins (Figure 14).

Figure 14. Pitcher plant (Nepenthes sp.) A and Sundew species (Drosera sp.) B, C (Photo credit: Gábor Solymossy)
Herbivory involves interactions for food resources between plants and plant-eating animals, which result in lowered density of plant populations and various types of injuries to individual organisms. Ultimately, flowering may not occur and seed production may suffer. Overgrazing is often very well observable in pastures (Figure 15). To defend themselves against herbivores, plants try to become less palatable and often produce toxins and stingy accessories such as thorns and spines. Strong hairiniess and thick cuticles may also be effective against small-sized herbivores such as the chewing insects. Leaves that are rich in fiber and less juicy are indigestible to many animals. In overgrazed pastures, noxious plants abound and the number of stingy plants increases.

Figure 15. The effect of overgrazing in a South-facing dry grassland (left) and a salt steppe (right)
Reiprocally advantegeous (mutualistic) relationships
7. 2. 5. Symbiosis and mycorrhiza
Relationships based on bilateral advantages are being established in order to ensure both partner’s success, mostly in the competition with others, or to uptake resources more efficiently. Such positive relationships are especially important for survival in extreme environments and at high population densities.
In the evolutionary history of plants, it was only the vascular plants who evolved nitrogen-fixing symbiotic associations, and organisms of these evolutionary lineages have significant competitive advantages. This happened because the nitrogen poor soils severely restrict the development and expansion of plants to new environments.
Each relationship that eases the utilization of atmospheric nitrogen by plants has unequivocal competitive advantages. In legumes (plants of the Fabaceae family), nitrogen fixation occurs due to nitrogen-fixing bacteria that live in their root systems. Within root nodules, bacteria called Rhizobium „manufacture” nitrogen, which is then readily available for uptake (Figure 16).
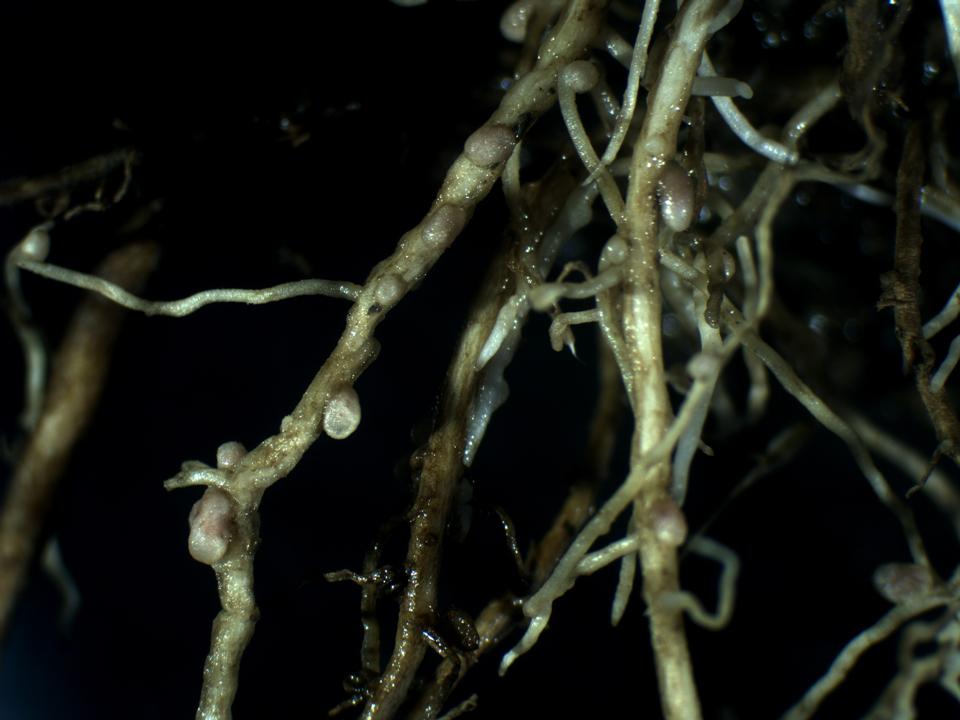
Figure 16. Root nodules on the root system of the White Clover (Trifolium repens)
Another well-known symbiotic relationship has evolved between fungi called Acinomycetales (see Chapter...) and different species of alder (Alnus). The alder species grow in nutrient-poor soils and consequently it is critical for them to have access to additional sources of nitrogen.
The symbiosis between fungi and algae has lead to another evolutionary success when lichens have appeared. Lichens are neither fungi or algae: they are a new group in the evolutionary history of plants, and have evolved as a result of a successful coexistence, the symbiosis. Neither the fungi nor the algae are able to produce lichen acids, and consequently this chemical compound constitutes the metabolic product of a new living entity. In this successful coexistence, the fungi use the nutrients produced by autotrophic algae during photosynthesis, whereas algae find a very good shelter in the web of fungus filaments. Some even believe that fungi use the trapped algae as a source of nutrients, which would point toward a rather parasitic relationships between the two parties.
Lichens are so successful that they can colonize the most barren rock surfaces or walls and can establish in the most extreme habitats from the arctic to the tropics, where neither the fungi nor the algae would be able to survive on its own (Figure 17).

Figure 17. Cup lichen (Cladonia sp.) and the Common Orange Lichen (Xanthoria parietina)
For a more efficient use of environmental resources, vascular plants establish mutualistic relationships with fungi as well. For example, mycorrhiza is a relationship between the root system of vascular plants and different species of fungi. A dense network of fungus filaments increases the surface of water and nutrient absorbtion by roots in nutrient poor soils. Some mycorrhizae even defend the roots from pathogens, and as a result the plant individuals involved in such relationships acquire higher viability. In exchange, the fungus partner has access to carbohydrates, and in addition plants help its development with vitamins, hormones and other growth regulators. More than 90% of the vascular plants establish mycorrhizal associations with various species of fungi.
Based on the position of the fungus filaments relative to the root structures, mycorrhizae can be divided into ecto- and endomycorrhizae.
In ectomycorrhizae the fungus filaments grow in a thick network on the surface of the roots, and penetrate only the outer layers of the root cortex. Opposite to that, in endomycorrhizae the fungi hyphae penetrate individual cells within the roots, where they form various structures.
Based on the shape and the type of structures generated by the fungus partners, several groups of mycorrhizae may be identified (Figure 18):
- in ectomycorrhizzal associations, fungi filaments form a mantle that covers the roots, and some penetrate in the intercellular spaces of the root cortex to produce a Hartig net of hyphae. This type of mycorrhiza is established mostly by trees, being present in many gymnosperms and deciduous trees. The fungus partner is usually a mushroom, which may be a basidiomycete or an ascomycete. In our forests, most mushrooms live in a mycorrhizal relationship.
- vesicular-arbuscular mycorrhiza (VAM), in which fungi filaments penetrate the cells and the intercellular spaces of the root cortex and produce baloon-like structures (called vesicles), snares or haustoria that resemble small trees. Species of disturbed habitats often have such mycorrhizae. In VAM, the fungus is only able to survive within the host plant cells.
- ericoid mycorrhiza has evolved in the heather family (Ericaceae), and is an endomycorrhizal association in which the fungi hyphae penetrate the rootlet cells, where they grow skein-like structures. Ericoid mycorrhizae enhance the nitrogen and phosphorus uptake in heathers, which typically live in acidic soils. The fungi are saprophytic.
- orchid mycorrhiza is typically found in the Orchid family (Orchidaceae), and it evolved to enhance the first phase of seed germination, because orchid seeds completely lack nutrient-rich endospermium. At later phases, the fungus-plant coexistence may shift to become a parasitic relationship.
A description of mycorrhizae is given in Chapter 4.
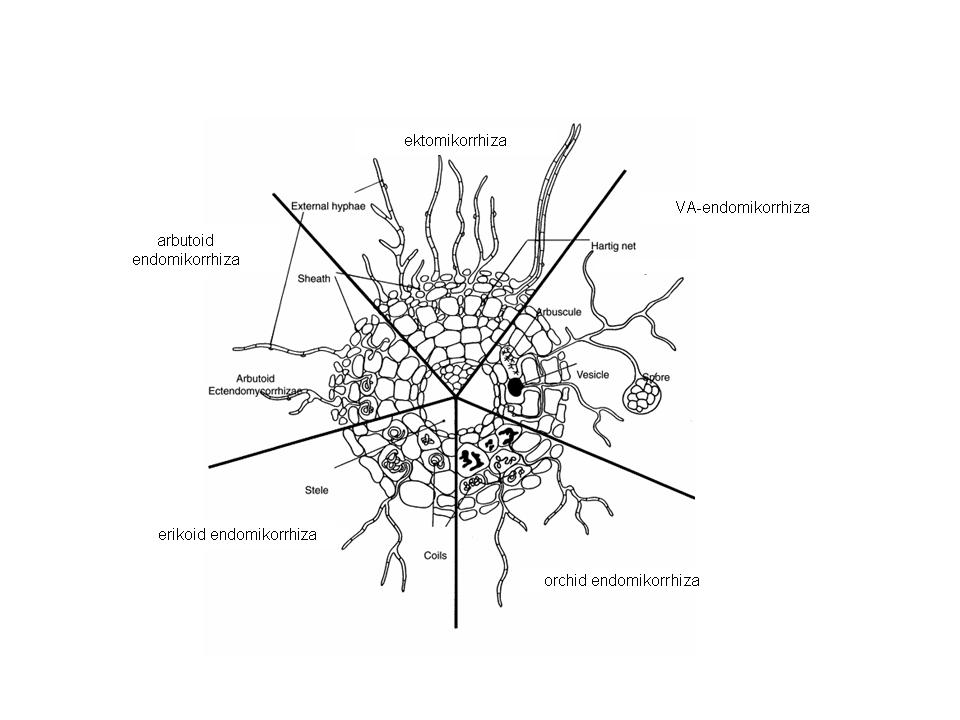
Figure 18. Graphical representation of four types of mycorrhiza
Mutualistic relationships have developed for a variety of other purposes in vascular plants. The pollination mutualism for example has evolved when plants, in exchange for various metabolic compounds provided to animals, use their services to become pollinated.
It is well-known that orchids and their pollinators have a very close mutualistic relationship. The plant doesn’t leave the pollination to chance and carefully places its pollen on a carrier, on whom it relies for pollination. On exchange, if the pollinator is able to find and recognize the plant, it gains access to a copious meal. The bee orchids (Ophrys) exploit the attraction of male insects to females to attract pollinators. Their lip, modifyied to mimic a female insect makes a perfect decoy for males, who will become pollen carriers following unsuccessful mating attempts (Figure 19). In the tropics most species from the Mimosoideae subfamily are pollinated by bats as their flowers have a big number of anthers consumed by bats. Pollination is done when bats visit these "multi-anthered" flowers.

Figure 19. False Spider Orchid (Ophrys arachnitiformis)
Some plants employ the cruellest tools to achieve their goals, and set up traps to their pollinators. Such are species of the Birthwort ( Aristolochia) genus, where the flower morphology develop a pitfall for the insects.
7. 2. .6. Commensalism
Commensal relationships are those in which the host partner is unaffected, but the commensal partner derives some sort of benefit.
For example, it has been long known that liana climb trees to gain access to more light. If lianas don’t occupy too much space (space parasite), trees are unharmed and are not affected in any way by the presence of lianas. On the contrary, a thorny, stingy liana may defend the tree bark against herbivore animals.
Some farmers apply the commensalism in their vegetable gardens. The squash is very often grown under the corn canopy because it does not withstand strong solar radiation. In this time, the large squash leaves cover the soil and prevent it from drying out. In exchange for providing shadow to the squash, the corn can grow on moister soils.
Commensal relationships are very diverse and are often the source of consistent coexistence of plants and animals, animals and fungi in the same habitats.
The evolutionary consequences of biological interactions
The close coexistence often produces evolutionary leaps. For example, following minor alterations in the flower structure, the pollinator animals change their feeding habits and as a result become separated and isolated from other populations of the same species. This ultimately leads to the development of new ecotypes.
If the new ecotype spreads, a new species may eventually emerge. Sometimes it is not clear which of the mutualist partners has evolved first. This type of simultaneous evolution is called coevolution.
An example is the relationship between fig species and their pollinators. Fig (Ficus) species, including the Commong Fig native to the Medierranean (Ficus carica) have a typical pot inflorescence which is pollinated only by fig wasps. The sole entrance to the inflorescence is readily recognized by its pollinators. None of them can grow without the other, as the fig wasps brings pollen to the flowers, but it needs the fleshy, nutrient-rich inflorescence to lay and grow its eggs. The fig fruit therefore represents a food source and protection for its larvae.
Gardeners know that fruits of the Common Fig that grown in Hungary are sterile, because inflorescences fall short of pollination. The geographic range of neither the fig nor the fig wasp does not reach Hungary, and therefore the planted fig trees remain unpollinated.
In the evolutionary history of defense and survival mechanisms, some other spectacular natural phenomena have developed in both animals and plants.
Mimicry is when some species try to be very similar to individuals of other species or to resemble its surroundings in order to become invisible to their predators. The most spectacular is the stick insect mimicry (Figure 20), but excellent examples of mimicry may be found e.g. in animals of open habitats in the arctic.

Figure 20. Stick insect (Photo credit Simig Dániel)
It often happens that weeds of cropfields try to mimic their crop neighbors, by taking up the shape of their shoots or the shape and the color of their fruitpods. Such is the relationship of the False Flax (Camelina) to the Common Flax (Linum usitatissimum), which has evolved during a long co-existence (Figure 21).
Figure 21. Common Flax plantation with False Flax plants
7. 3. Plant communities and community dynamics
The niche theory posits that habitats are shared by those populations to whom environmental conditions are optimal (i.e., each environmental factor is within the tolerance limits of each population). Of course, one habitat is always suitable to populations of multiple species, and consequently organisms fiercely struggle for resources. The fight, which is regulated by biological interactions, eventually leads to a community of populations in which niches don’t overlap or only partially overlap, and populations are settled for a long-term coexistence.
Populations of many species will try to survive within the same habitat, and because of the competition, they will divide their niche spaces very often. Niche space division (i.e., niche segregation) may affect multiple niche dimensions. When the split niche dimension is the time, niche segregation may be seasonal or diurnal.
The seasonal niche segregation can be observed very well in the understory of deciduous forests. In the springtime, when a sufficient amount of light reaches the understory, the forest is populated by light-demanding herbaceous plants. These plants are good at coping with cooler soils. Later on, when the tree canopy closes, these plants disappear (withdrawn to bulbs, rhizomes and tubers until next spring), giving way to more warm-demanding, but shade tolerant species. The early spring aspect is usually more colourful and full of flowers, because the plentiful light and the moist soil represent and ideal environment to many plant species (Figure 22).

Figure 22. Summer (upper) and early spring (lower) aspect of an oak-hornbeam forest (Photo credit J. Bölöni)
The niche segregation can also happen along the day. This phenomenon is more obvious in moving animals. Perhaps everyone knows that a wasteland is the hunting ground of the Common Buzzard during the day, and of the Long-eared Owl during the night.
We have seen that one of the consequence of niche segregation is the ability of habitats to sustain many populations, but only those to whom the environmental factors lay within their tolerance interval. Many populations try to „adjust” their niches to the habitat they live in, and as a result they develop adaptiv traits. We say that a colonizing population is continually framing its environmental tolerance limits to its environment, and these fine alignments eventually lead to ecological adaptation.
The community of populations in a habitat has attained a state of equilibrium if competitive interactions have calmed down, positive interactions have strenghtened, niches segregated and niche-gradients developed. Populations of a stabilized plant community never share similar niches.
Thanks to a fine division of niche spaces, populations of several species can ultimately establish plant communities (phytocoenoses). Because plants are sedentary, the appearance of plant communities is relatively constant, and can produce specific habitat patterns (Figure 23. 24).
A plant community – (also called plant association) – is therefore:
- spatially repetitive, has a constant species composition and constant appearance
- has a well-defined structure
- always develops under similar environmental conditions

Figure 23. Beech forest in Magas- Bakony of the North Hungarian Mountains
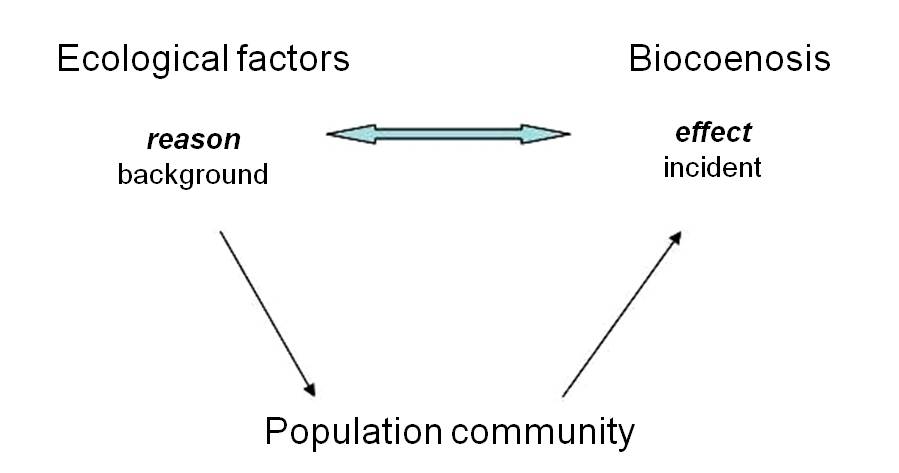
Figure 24. Diagrammatic representation of the development of plant communities
Plant communities are the basic units of vegetation. They are composed of populations of species that appear more or less uniformly across the vegetation units, called characteristic species.
For example, in the Central Mountains of Hungary, beech forests have developed above 600 m of elevation. The characteristic woody species of these submontane and montane beech forests is the European Beech (Fagus sylvatica).
A typical plant association of the Pannonian salt steppes is the Achilleo-Festucetum pseudovinae. Both Achillea asplenifolia and Festuca pseudovina are characteristic species in this community. The scientific name of the plant communities derives from the name of the two characteristic species.
Several contrasting views of the plant community assembly have developed in time. The differences consist in the interpretation of the spatial boundaries of a plant community, and its inner dynamics.
1. According to F. I. Clements, groups of populations coexist under similar environmental conditions – „they always occur together” – and because they arrange along a niche gradient, ultimately build up structured, well-organized associations.
2. According to H. Gleason, the link between different populations that share a habitat is loose and dictated by environmental conditions at a specific point in time. As a result, plant associations are less structured than previously thought. The boundaries between different plant communities are blurred and several types of transitions exist.
Plant communities are dynamic units and although their boundaries are often well outlined, they may change in time, and in addition the participating populations may even be replaced.
The community dynamics has a spatial and a temporal dimension. In the spatial dimension, changes may be horizontal (produce spatial vegetation patterns) and vertical (produce vertical vegetation structure). In the temporal dimension, changes may be seasonal (such as the seasonal aspects of a forest) and pointing forward (such as the vegetation succession).
7. 3 .1. Succession
The term succession describes the transition betwen two plant communities over time. Transitions occur following changes in the environmental conditions and because of inner structural rearrangements in the community.
We can distinguish different types of succession:
- secular succession – happens over extended geological times, is not repetable in time, and follows big climatic changes
- biotic succession – occurs in short time intervals, under the same macroclimatic conditions, and is always a repetitive process. It follows fundamental changes in the environment, which may be triggered by
endogenous factors (e.g., hummus formation)
exogenous (allogenous) factors (e.g., river bars following floods)
F.I. Clements, regarded as the father of the succession theory, delineates six basic processes during succession. These are: nudation (stripping to bare ground), migration (arrival of propagules), ecesis (establishment and growth of new populations), reaction (organisms affect their physical environment and modify it), competition (struggle for resources), stabilisation (attainment of a well-organized final community).
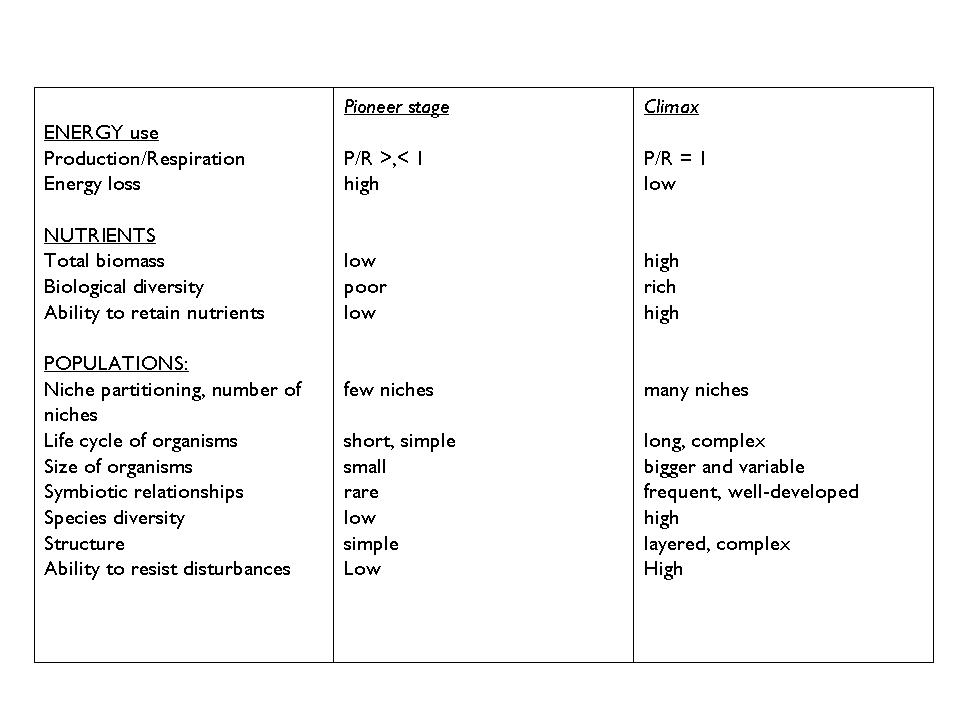
Each biotic succession encompasses an initial stage (pioneer stage) and a final stage (climax).
Several intermediary phases (called seral stages) exist between, which may differ substantially with regards to their production and energetics.
The final, climax stage is the stage of stabilization, which is governed by a dynamic equilibrium. Communities in the climax stage have the highest productivity and also the spatially most complex, layered structure. Consequently, they have very efficient biogeochemical cycles and energy flow. The interactions between the component populations are manifold and complex (Table 1).
Table 1. Comparative description of pioneer and climax plant communities (Modified from H.T. Odum and P. Juhász-Nagy)
Different types of succession
→ primary (soil not present from the start): e.g. on moraines following deglaciation, new riverbars and cooled down lava flows (Figure 25)
→ secondary (soil is present from the start and is formed of decaying vegetation remains): e.g., on ploughlands, forest clearings or following big floods (Figure 26)
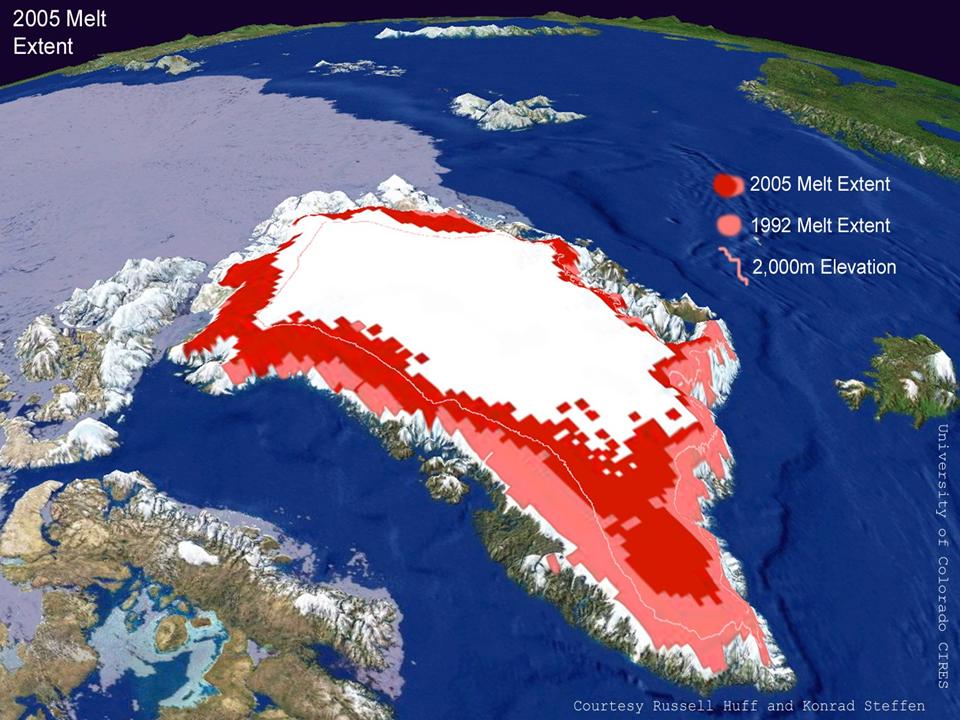
Figure 25. Snapshots of the melting icesheet on Greenland (http://cires.colorado.edu/science/groups/steffen/greenland/melt2005/)
In Hungary, primary succession stages are typical to sandhills and falling loess walls. They have been expressively described by Gábor Fekete (1992). Thanks to localized primary succession events, two different substrates may produce two radically different plant communities even under similar climatic conditions. Such is the case of the extrazonal sandhill vegetation within the zonal loess vegetation.
Succession on sand dunes
(see the movie „Sand vegetation in the Duna-Tisza region”)
- Sand is a coarse-grained sediment and a typical component of poor soils.
-Sand dunes are extreme habitats with low nutrient content and poor water retention ability.
- Most species have broad ecological tolerances, they are not specialists
-The resource share between different species’ populations is strongly unbalanced
-Vegetation succession is not linear: because of the continuous disturbance, dune grasslands are long-lived and bush encroachment never happens
-Species are drifting, and sand habitats gather mostly generalist species from „nearby”
- Climax stages are never reached, or at least it is unclear how they might look like. The White Poplar-Juniper woody vegetation that wee see today on the Pannonian sand dunes do not bear by all means the characteristics of end-point plant communities.
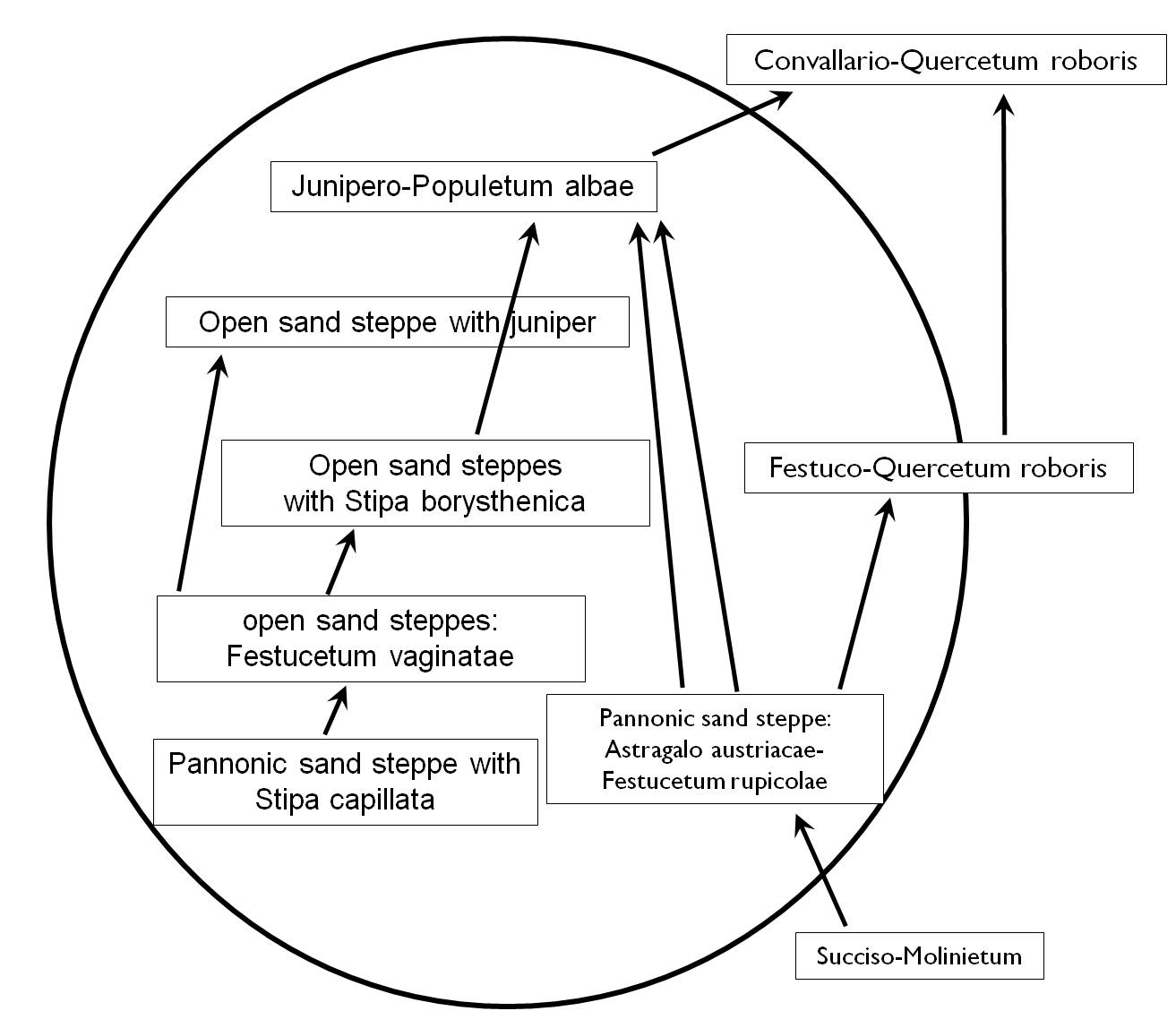
Figure 26. Schematic representation of edaphic succession on sand (G. Fekete 1992).
- Dry mesic oak forests
- White Poplar-Juniper communities
- Open perennial Hungarian Fescue grasslands with Juniper
- Open sand grasslands with Stipa borysthenica bunchgrass
- Open perennial Hungarian Fescue grasslands
- Steppic sand grasslands with Stipa capillata bunchgrass
- Sand steppe grasslands
- Molinia (Purple Moor Grass) wet meadows
- Hungarian Fescue – Oak savannas
Succession on loess
(see the movie „Loess vegetation along the Danube”)
- Loess is very favorable to soil formation, the soils have a high nutrient content and a good water balance.
- Species are generalists at the beginning, but there is a precize niche segregation even in the starting successional stages, i.e., in the sand steppe communities.
- The vegetation development is linear and advances from pioneer stages towards the climax community.
- The loess vegetation has its own, indicator species - which may be woody.
- Climax communities are clean-cut and species-rich, and in Hungary they are represented by the Submediterranean Tatar Maple-Oak loess communities.
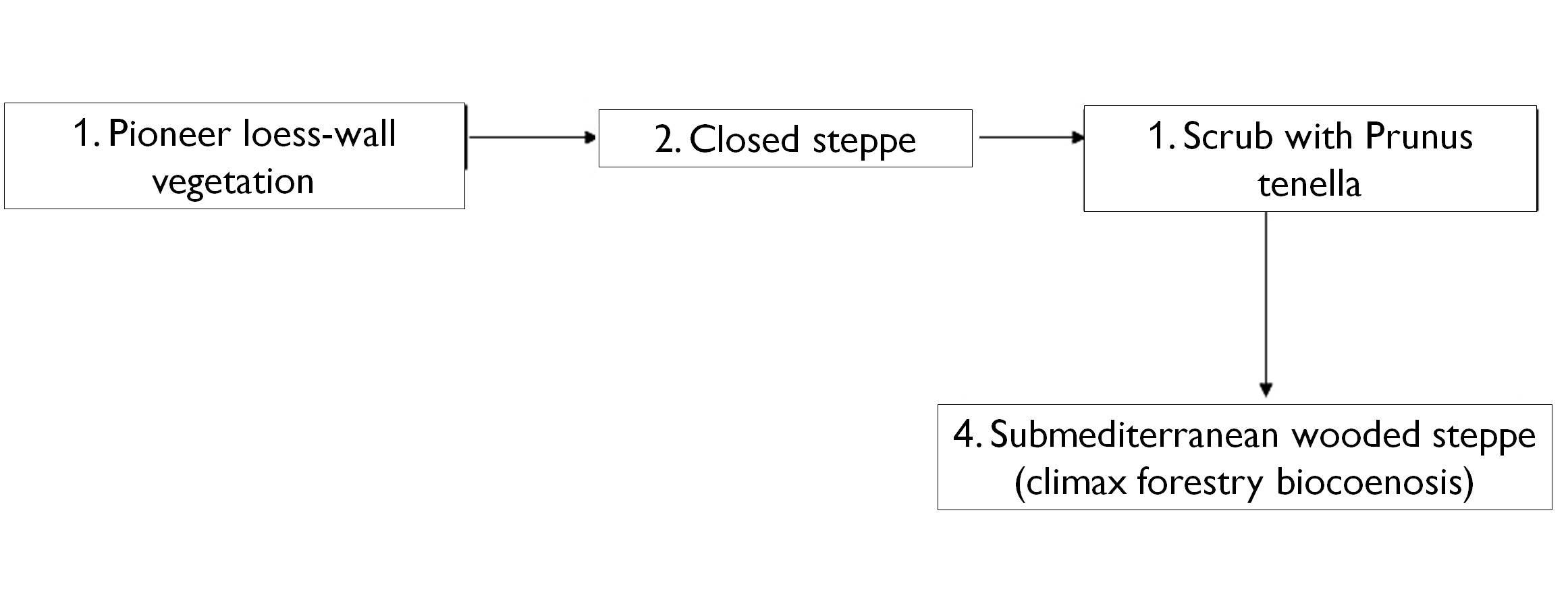
Figure 27. Schematic representation of succession on loess (G. Fekete 1992)
- Pioneer loess wall vegetation
- Closed steppe grassland
- Dwarf Russian Almond bushes
- Submediterranean forest-steppe
- end-point (climax) forest community
A significant part of the loess grasslands of Hungary has been converted to agricultural lands. However, secondary succession on abandoned ploughlands might have the ability to regenerate loess steppes, provided a sufficient number of viable propagules is still available in the soil (Figure 28.). Secondary succession often can be seen on abandoned mine tailigns (Figure 29.).

Figure 28. Abandoned ploughland colonized by annual species (e.g., Papaver rhoeas) in a loess habitat on the Hungarian Plain (Photo credit..)

Figure 29. Uncovered bare surface in an open bauxite mine in Gánt (Vértes, Hungary) (Source: http://www.karpat-medence.hu)
With respect to its direction, succession may be:
→ progressive or developing, which entails increasing structural complexity and stability of plant communities
→ retrogressive or receding, which leads to degraded stages and may ultimately trigger the collapse of plant communities. Good examples are the continuously deforested areas where, because of accelerated soil erosion, new colonizers live in unstructured communities for a long time. We may see very often such degraded forests in the Mediterran areas.
The speed of succession always depends on the following:
- substrate type
- climatic factors
- distance from the propagule source
We have seen that community dynamics can have two dimensions: spatial and temporal. So far, we have given examples of temporal dynamics (i.e., temporal succession). Spatial succession (also called zonation) is when distinct successional phases develop next to each other because of abruptly changing environmental conditions. Good examples are the littoral zones of rivers and lakes (Figures 30, 31).
1. Gravel bar vegetation: annual pioneer species and Purple Willow communities (Salicetum purpureae)
2. Purple Willow and Reed communities
3. Riverside scrub with Almond Willow (Salicetum triandrae)
4. Soft wood (or Willow-Poplar) riverine gallery (Salicetum albae-fragilis)
5. Pannonic alluvial forests – with Black Alder and Hungarian Ash (Fraxino pannonicae-Alnetum)
6. Hardwood (or Oak-Ash-Elm) gallery (Fraxino pannonicae-Ulmetum)
7. Continental oak forests
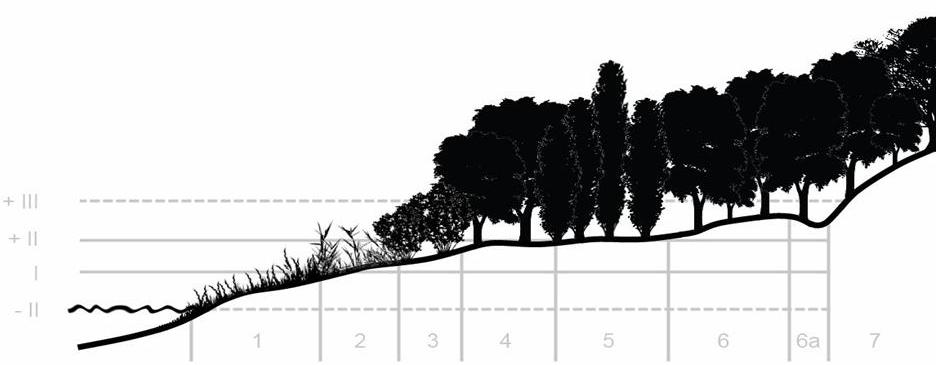
Figure 30. River zonation (From I. Csűrös) with different flood levels marked.
1. Free-floating macrophyte communities
2. Fixed aquatic macrophyte communities
3. Reed community
4. Tall sedge communities
5. Marsh- and wet meadows
6. Woodland with Grey Willow (Salicion cinereae)
7. Willow meadows – with Gray Willow (Salicion cinereae)
8. Alluvial forests with Black Alder (Alnion glutinosae)
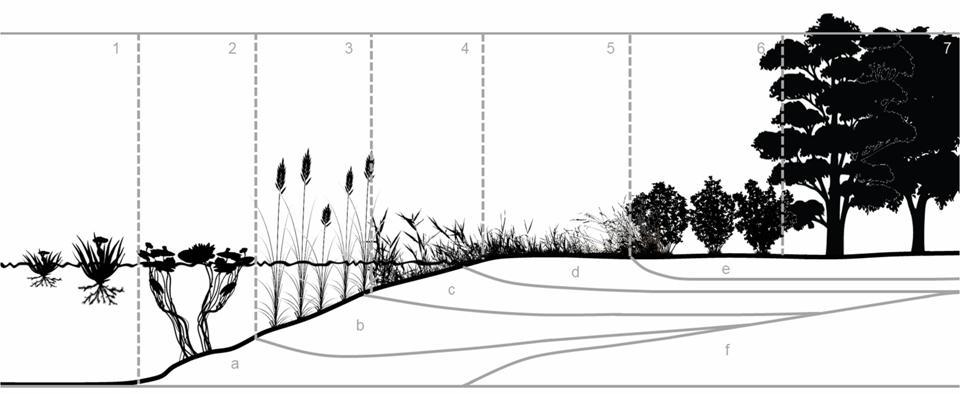
Figure 31. Standing water zonation (From Soó R., 1933.)
Documenting vegetation succession in either natural or cultivated ecosystems may substantially contribute to preventing retrogressive succession (i.e., degradation) and enhancing progressive vegetation processes. The latter are much more cost-effective when it comes to maintaining ecosystem functioning, and may be critical to the formation of sustainable, livable natural landscapes.
References
- Cain, M., Bowman, V.D., Hacker, S.D. 2011. Ecology. (2nd ed). Sinauer Associates Inc.
- Fekete G. (szerk.) 1985: A cönológiai szukcesszió kérdései. Biológiai Tanulmányok 12. Akadémiai Kiadó, Budapest.
- Mátyás Cs. 1997: Erdészeti Ökológia. Mezőgazda kiadó, Budapest.
- Fekete, G., Molnár, Zs., Horváth, F. 1997: A magyarországi élõhelyek leírása, határozója és a Nemzeti Élõhelyosztályozási Rendszer. Nemzeti Biodiverzitás-monitorozó Rendszer II. MTA-ÖBKI - KTM kiadás, Budapest.
- Pásztor E., Oborny B. (szerk.) 2007: Ökológia. Nemzeti Tankönyvkiadó, Budapest.
- Simon T. (szerk.) (2001): Növényföldrajz, Társulástan és Ökológia. Tankönyvkiadó, Budapest.
- Török p., Tóthmérész B. (2006): Növényökológiai alapismeretek. Kossuth Egyetemi kiadó, Debrecen.
- Turcsányi G. (szerk.) 1998: Mezőgazdasági növénytan. Mezőgazdasági Szaktudás Kiadó, Budapest. 2. kiadás



