Plant organs (Organography)
Author: Zsolt Erős-Honti
3.1. Root
3.1.1. Evolutionary origin, ontogeny and architecture of the root
The main functions of the root are absorption of nutrients (water and minerals), anchorage of the plant and in case of perennial species, the storage of organic compounds during the dormancy so that to provide nutrients for sprouting at the beginning of the next vegetation period when chlorophyll-less plant is not capable of photosynthesis.
In the evolutionary sense, root is the youngest organ of the plant; however, it is the first to develop during the ontogeny. Its evolution was forced by the fact that in terrestrial environments nutrients can only be uptaken from the soil (not through the whole plant surface as in water), thus a specialised absorptive organ capable of acquiring the limited amount of minerals and water was necessary for the survival. The first plants with real tissues, the pteridiophytes had only adventitious roots deriving from the shoot, because their embryo had one meristematic pole, the plumule (unipolar embryo) that developed into the shoot. Bipolar embryo with radicle first appeared amongst the earliest gymnosperms, so they were the first plants bearing a real taproot.
Taproot is the root developing directly from the radicle of the bipolar embryo. It should be distinguished from the adventitious roots deriving from any part of the shoot (i.e. above the cotyledons). In case of taproot system, the radicle of the embryo develops into a dominant primary root (taproot) and it gives rise to secondary root branches (lateral roots). In contrast, the radicle of some species cease to grow early during the ontogeny (or even it may die) and its function is taken up by adventitious roots (fibrous roots) originating from the stem at height of the cotyledons ( mesocotyl) or close to this region (fibrous root system). Adventitious roots also derive from the upper regions of the stem, often on the mature plant. Such roots are to be observed on rhizomes, stolons or cuttings. In both types of root systems, the thinnest root branches serving the function of nutrient absorption are called hairy roots.
3.1.2 The functional zones and primary tissues of the fine root
On the longitudinal axis of the fine roots, different zones can be distinguished according to their structure and function. Zone of cell division (root tip) contains the meristems, where the majority of the cells are born by the mitotic activity of the cells. In the centre of this zone, promeristematic cells (initials) are located. Among the initials, a group of cells with low mitotic activity are to be found; this is the quiescence center (central mother cells). According to the histogen theory, derivatives of initials differentiate into primary meristems. In eudicots (Rosopsida) the outermost layer of the meristematic zone is the dermato-calyptrogen that produces the rhizodermis and the root cap 1. Above the central initials, toward the root collar, in the axial region of the root is the plerome that forms the cells of the stele. In a cylindrical manner, plerome is surrounded by the periblem, which produces the tissues of the primary cortex.
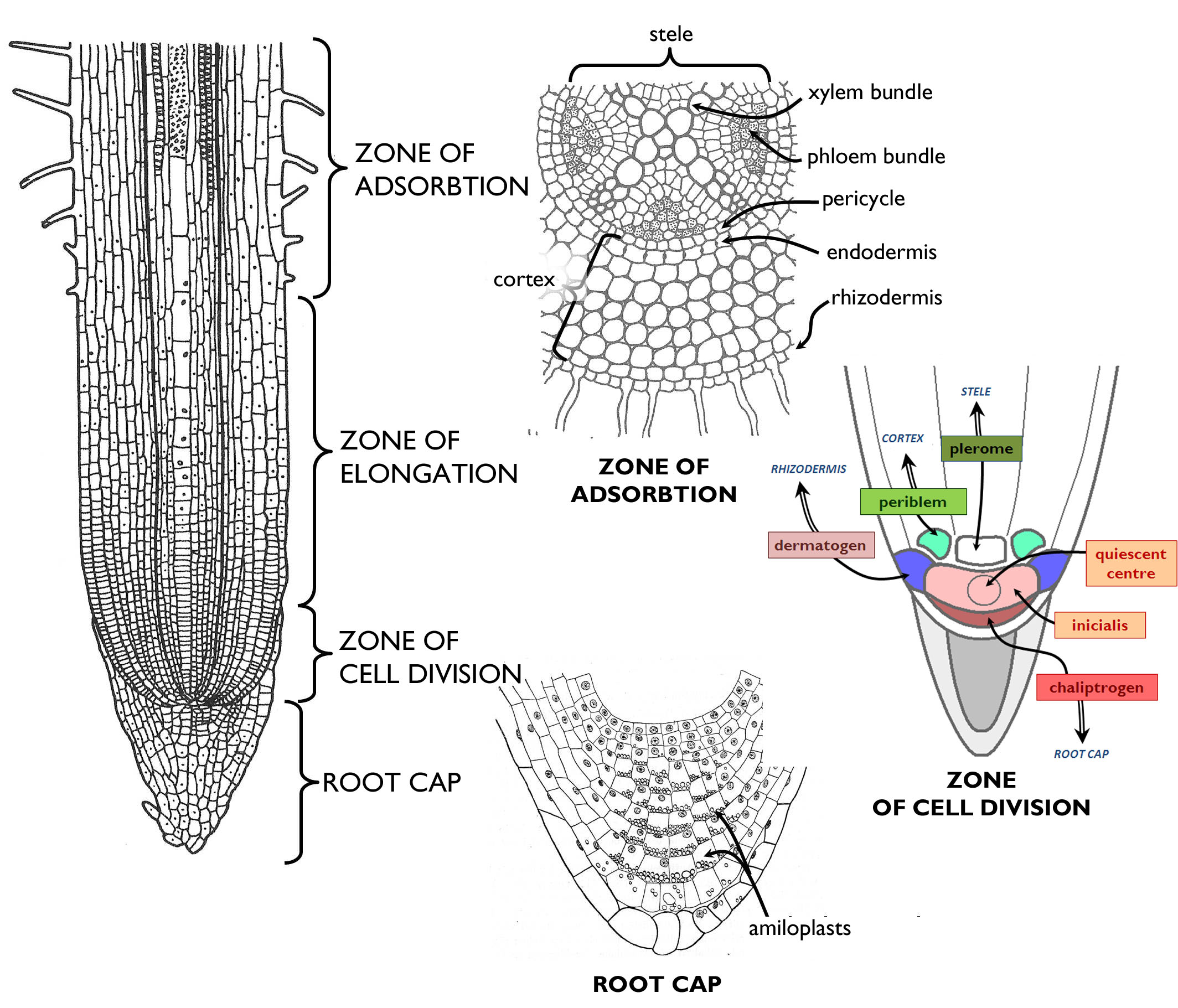
(Based on the figures of Jacob et al. and those by Habelrandt)
The tip of the root is covered by a mass of parenchymatic cells, the root cap (calyptra). In its innermost, central region mitotic cells are present (columella). The main function of the root cap is to protect the root tip from mechanical damages (that would cease root growth). Cells of the calyptra secrete different compounds into the environment, and the cells adjacent to the soil particles become slimy and finally tear off, what aids the growing of the root within the soil. By special amyloplasts within its cells, root cap is responsible for sensing the direction of gravitational force and thus the gravitropic movements of the root.
The next region of the fine root above the zone of cell divisions is the zone of elongation. In this area, the cells of the root elongate by an intense water uptake. (Consequently, this is the zone of the most pronounced longitudinal growth.) Together with cell growth, differentiation also starts here: at the end of the zone primary tissues are found.
In the next zone, differentiated tissues begin to function. This region is called zone of absorption (in angiosperms: zone of root hairs ). This is the area of nutrient uptake: minerals and water here enter into the root cortex and then into the bundles of the stele. Tracheary elements finally transport them into all other parts of the body. Root hairs continuously decay at the upper region of this zone of some millimetres, i.e. the rhizodermis tears off. This is the beginning of the zone of transportation. Here, secondary tissues and lateral roots are produced and thus this area is also called the zone of secondary growth or the zone of root branching.
Primary tissue structure of the root is most clearly observed on cross sections made of the zone of adsorption. The inner substance of the organ surrounded by the rhizodermis can be divided into two main regions (similarly to the stem): the central part containing the vascular bundles is the stele that is surrounded by the primary cortex. Through the root hairs, ions are taken up by active transport, while water is by osmosis. From the rhizodermis, uptaken materials get into the outermost cell layer of the cortex. Should these cells differ anatomically form the other cortical cells, we talk about exodermis. Such layer is present chiefly in roots living in moist or drowned soil. In the secondary wall of exodermal cells suberin is deposited, and thus these cells comprise a waterproof barrier for apoplastic transport. Most frequently the cortex is composed of storage parenchyma. The innermost layer of the cortex, adjacent to the stele is the endodermis. Similarly to the exodermis, cell walls of this row of cells are suberinised, so it serves as an inner barrier impermeable for water on the borderline of the stele and the cortex.
The outmost layer of the stele is composed of cells retaining their meristematic activity – this is the pericycle, the region of lateral root initiation. Besides, these dividing cells take part in the formation of the vascular cambium and the phellogen, both producing secondary tissues of the thickening root.
The ground substance of the stele is parenchyma, in which simple bundles are embedded. Considering the number of xylem bundles, diarch, triarch, tetrarch, pentarch etc. roots are distinguished. In general, the root of monocots (Liliopsida) is polyarch, i.e. it contains more than 8-10 xylem bundles, and these bundles do not attach to each other in the root axis (so the root centre is filled with pith parenchyma). To the contrary, in the root of eudicots (Rosopsida) less xylem bundles occur (oligarch root), and these bundles are usually connected to each other comprising a star-like structure in the centre of the cross sections.
A characteristic feature of the xylem bundles of the root is that the primarily initiated elements (protoxylem) face outside, while the youngest tracheary elements (metaxylem) are on the inner side of the bundles (exarch xylem). Phloem bundles are positioned between the xylem ones, slightly outwards from them. In these bundles protoelements also face toward the rhizodermis and the metaphloem lies on the inner side (exarch phloem). Supportive tissue elements (principally sclerenchyma fibers) are attached to the xylem bundles, so the supportion of the organ is concentrated at the longitudinal axis. This cable-like structure is the most advantageous one for resisting against pulling forces in the anchoring root, without depositing too much excess material.
3.1.4. Secondary thickening of the root
The root of almost all eudicots (Rosopsida) thickens secondarily. (This is the reason why the root collar is the thickest region of the root.) However, roots of monocots (Liliopsida) do not go through such process. In these roots, the protective function of the decaying rhizodermis is taken up by the exodermis, while the innermost cell layer(s) of the cortex develop into a close inner barrier.
At the upper region of the zone of adsorption, the root hairs disappear and the parenchyma cells between the xylem and phloem bundles of the stele dedifferentiate and regain their mitotioc activity. The stripes of meristematic cells formed between the ribs of primary xylem bundles and inside of the phloem strands attach to each other and later they are completed into a continuous cambium layer with the bands of pericycle adjacent to the xylem bundles. Due to its origin, the cross section of the vascular cambium2 is characteristically sinuous in the root (Figure 2). Cambial cells of the root divide periclinally (similarly to those of the stem) and the daughter cells cut off to the exterior differentiate into secondary phloem, while those produced inwards turn into secondary xylem elements. As a result of the deposition of secondary tissue layers, the vascular cambium gradually attains a circular shape in cross section.
The innermost region of the thickened root contains the primary xylem bundles (forming a star-like structure in oligarch roots). Outward from these are the layers of the secondary xylem (the youngest layers being in the outermost region, adjacent to the cambium). The structure of the secondary xylem of the root is quite similar to that of the stem: it may have annual (growth) rings within and radially oriented rays connecting the xylem elements to the phloem. The youngest members of the secondary phloem are in the vicinity of the vascular cambium, while the oldest ones are shifted below the decaying cortex. Primary phloem bundles cannot be observed in the thickened root, because these thin-walled elements are compressed and thus disappear.
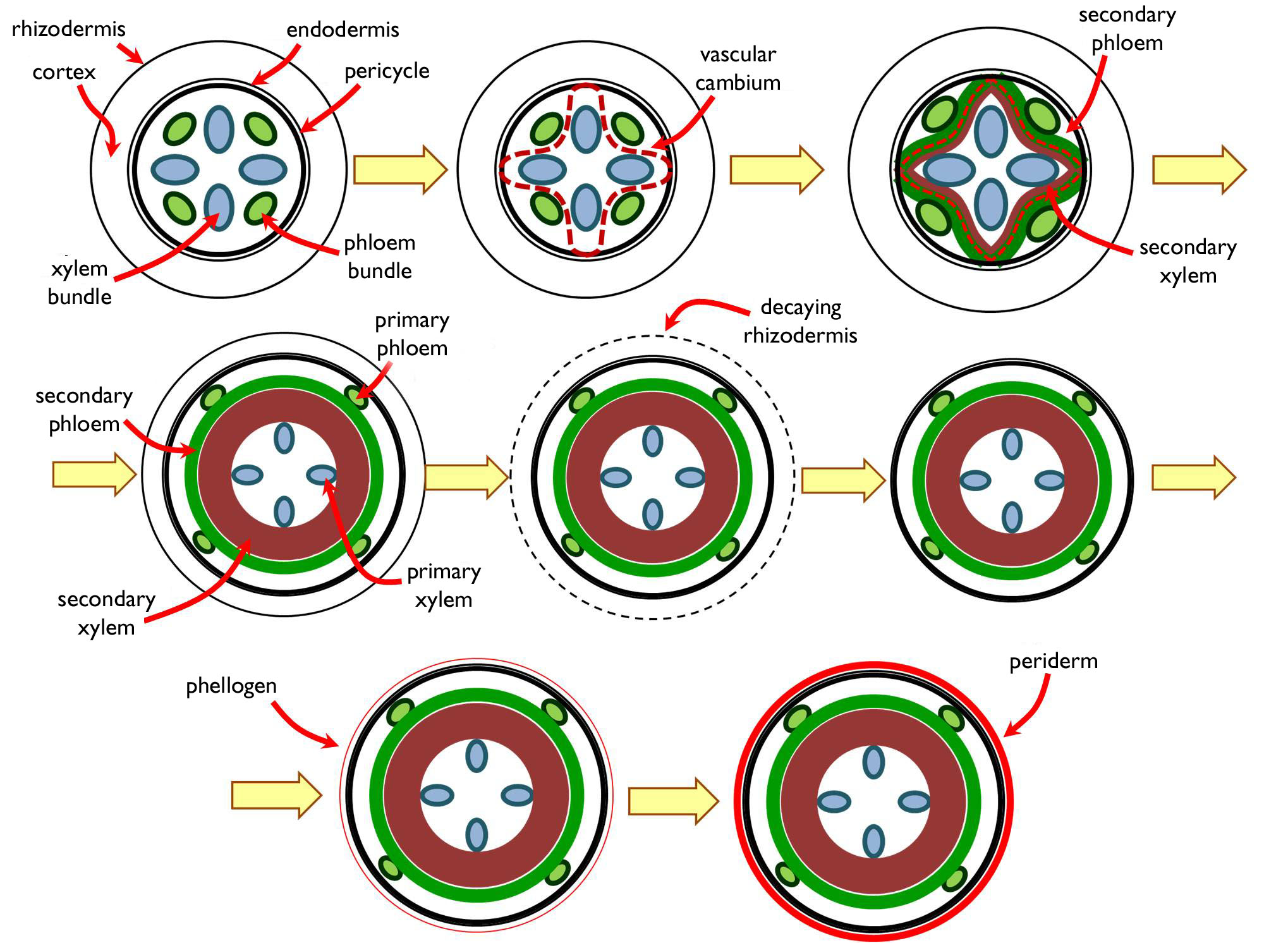
Figure 2. Secondary thickening of the root.
To the outside from the secondary conductive tissues, the remains of the primary cortex are observable for some time. However, this tissue layer is incapable to extend while diameter is increasing, thus it tears off quite early. Later, in the same position a secondary protective tissue, the periderm develops. The meristem producing the periderm (phellogen) is composed of the pericycle cells not taking part in cambium formation and the inner cortical parenchyma cells that are adjacent to the xylem bundles (since these regions of the pericycle took part in production of the vascular cambium). The strands of these dual origin attach to each other and form a continuous meristematic cylinder, the cork cambium (phellogen) 3. Phellogen may be either monopleuric or bipleuric. Periclinal cell divisions of the monopleuric phellogen produce the phellem to the outside. In addition to phellem, bipleuric phellogen forms also a thick phelloderm to the interior. Phellem usually consists of parenchymatic cells and functions as a secondary storage cortex.
3.1.5. Ecological adaptation of the root
In addition to its basic functions, root may serve other adaptive purposes, as well. Taking up a new function naturally requires specialised anatomical structure. Storage roots (taproot, tuberous root) have a well-developed secondary cortex of storage parenchyma in the zone of secondary growth. The cortical cells accumulate nutrients in the form of starch stored in leucoplasts. Storage compounds are utilised at the beginning of the next vegetation period for the development of the germinating stem (e.g. Daucus carota subsp. sativus, Dahlia × variabilis). A special type of storage root is the succulent root (e.g. Chlorophytum comosum) that bears water storage parenchyma in the secondary cortex.
Aerial roots being exposed to light have the possibility to produce organic matter via photosynthesis (green photosynthetic roots). In case of these organs, the outer cell layers of the root cortex turn into a chlorenchyma. Some aerial roots are also capable of adsorbing water from the vapour of the air (e.g. epiphytic orchids or Monstera deliciosa). Their root is covered by a special multi-layered rhizodermis called velamen radicum. (For details see Chapter 2.) Supportive roots (e.g. knee roots, brace roots) are to resist bending forces, thus their anatomical characteristics are the thick clusters of fibers in the cortex, close to the epidermis. These roots have a large stele with extended pith parenchyma and a quite thin cortex with the vascular bundles and sclerenchymatic fibers. The adaptive histological feature of pneumatophores is the vast aerenchyma, chiefly in the cortex, yet sometimes also in the pith.
Roots, serving ecological interactions between a plant and another organism, have anatomical features adapted for nutrient transport. Structure of haustorial roots does not differ considerably from that of the ordinary root with the function of nutrient uptake, yet the hairy roots do not take up the compounds through root hairs, but they penetrate the conductive tissues of the host as suction threads (haustoria). Holoparasites root in the phloem, while the roots of hemiparasites absorb water and ions from the xylem of the host.
In symbiotic roots, the mutualistic partner may live either in the intercellular cavities or even within the plant cells. Several plant species live in mutualistic interaction with microbes capable of nitrogen fixation. Only prokaryotes can convert the nitrogen gas (N2) into other nitrogen forms (organic nitrogen, ammonia) that is utilisable for the plant. Cyanobacteria live within the coralloid roots of species of the phylum Cycadophyta, where they comprise a continuous bluish layer in the cortex. A similar layer is formed by Frankia species, belonging to the phylum Eubacteria, in the cortical region of the actinorhizae of different alders (Alnus). The best-known type of mutualism between nitrogen fixing organisms and plants are the root nodules of the legume family (Fabaceae), inhabited by Rhizobium bacteria. The bacteria get into the cortex of the fine root via an infection thread formed within the root hair. In the cortex, bacterial cells produce hormone-like compounds that induce cell proliferation resulting the development of the nodule. In the nodule, bacteria do not live intercellularly but within the plant cells, like a certain type of organelle ( symbiosome)4.
The absorptive organ of symbiotic origin between plant roots and fungal hyphae is called mycorrhiza. More than 90% of plant species live in such a mutualistic interaction with fungi. Two main types of mycorrhiza are distinguished based on their anatomy. In case of ectomycorrhizae, fungal hyphae form a continuous cover (fungal mantle) on the surface of fine roots, where the root hairs are consequently missing. From the mantle, hyphae grow among the epidermal and cortical plant cells surrounding them and serve as the surface of nutrient exchange between the symbiotic partners (interface). This inner system of hyphae is the Hartig net. Outwards from the fungal mantle emanating hyphae run among the soil particles and so increase the absorptive surface of the root (which is much larger than the total surface of the missing root hairs). Consequently, the fungal partner supports water and mineral uptake of the plant, while the fungus gets organic compounds from the plant in exchange. (Knowing the role of ectomycorrhizae, obvious is the fact that the presence of the fungal partner is crucial for the survival of gymnosperms lacking root hairs.)
The other anatomical type of mycorrhizae is endomycorrhiza. In this case, hyphae do not form a continuous mantle on the root surface. Instead, they penetrate into the (cortical) plant cells where they form characteristic fungal structures. (It is worth to mention, that hyhae break through only the cell wall, the cell membrane is only invaginated. Thus, the plant cell remains alive.) The most widespread type of endomycorrhizae is thevesicular-arbuscular mycorrhiza. In this mutualism, hyphae develop highly branching, tree-like haustoria (arbuscules) and vesicles within the plant cells. Arbuscules serve as the interface between the mutualistic partners, while vesicles are storage structures. Similarly to ectomycorrhizae, emanating hyphae grow into the soil also from the endomycorrhizal roots, with the same function as described above.
3.2. Shoot
The shoot is the unity of the vegetative organs (stem and leaves) developing from the embryonic shoot apex (plumule). In the history of vascular plants, this was the first organ to evolve.
3.2.1. The ontogeny of the shoot
The organs of the shoot originate from the shoot pole of the embryo (plumule). In case of perennial plants, if vegetation periods are separated by intervals of dormancy caused by the periodically unfavourable climatic conditions, growing of the shoot apex cease from time to time. The meristems survive the harsh period in a dormant form. This incipient, embryonic shoot of short internodes, being in the stage of dormancy is called bud. Buds may be at different positions on the plant – according to the ecological environment and the survival strategy of the plant. Based on bud position, Chriten C. Raunkiaer distinguished different life forms (in details see in Chapter 6!).
The bud is composed of the following structural elements: the meristems of the dormant shoot, the primordial shoot axis (bud axis), primordial leaves covering the axis and (usu.) specialised leaf scales surrounding the previous structures (bud scales). Besides, reproductive (flower buds) and mixed buds contain incipient flowers, as well.
At the beginning of the vegetation period, the meristems of the shoot apex activate and so the shoot begins to grow. Similarly to the fine roots, the shoot also can be divided into zones of different stages of development along the longitudinal axis (Figure 3). On the uppermost portion is the embryonic zone of the shoot that is the entirety of the initials and the primary meristems of the shoot tip.
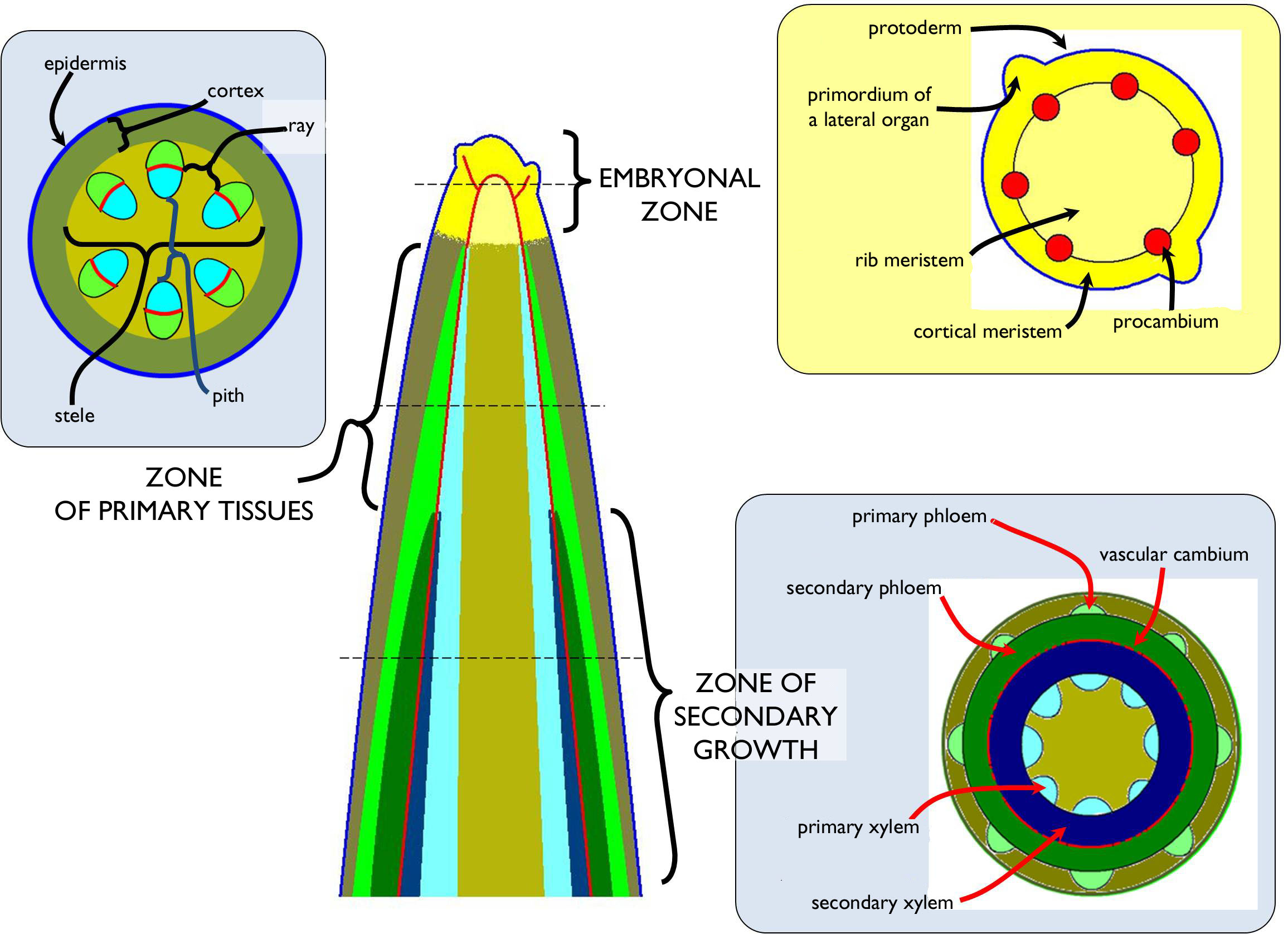
Figure 3. Zonation of the shoot.
Below the meristematic region of the shoot apex, the cells produced by the dividing tissues become elongated and begin to differentiate ( zone of differentiation). In the next zone, the primary tissues of the stem are present (zone of primary tissues). In most types of the stem (both in herbs and woody plants), primary growth is followed by the process of secondary growth that has two different types. Secondary lateral meristems (vascular cambium, phellogen) are responsible for the increase of the diameter (secondary thickening). Besides, in the nodes of some non-woody stems (e.g. in grasses or sedges) meristematic cells capable of further division remain. Their function results subsequent elongation of the shoot axis (intercalary growth).
3.2.2. Anatomy of the shoot axis
3.2.2.1. Primary tissues of the shoot axis
The principal role of the stem is to hold the other organs of the plant as an axis of the body, but it also takes part in nutrient translocation between them and – in most cases – it is also capable of photosynthesis, when young. On the outer surface it is covered by an – usu. single-layered – epidermis bearing trichomes, stomata and cuticle. Similarly to the root, the stem can be divided into two distinct tissue regions, the primary cortex and the stele. In stems being above the soil surface has an insignificant cortex of few cell layers.
The stele being in the centre of the stem contains the vascular bundles. In contrast to the root, this region is not so clearly separated from the cortex, since no conspicuous bordering cell layer (like endodermis) occur in the majority of the stems, it is merely defined by the outermost conductive elements of the bundles. If no vascular bundle is present in the axis of the stem, this region is either filled with pit parenchyma or it is hollow due to a large intercellular space opening within. Among the conductive elements, radially arranged rows of ground tissue cells are present; these are the rays.
The stele of the stem always contains complex bundles. If these bundles are collateral, the xylem faces to the centre of the stem, while to phloem part toward the epidermis. In the xylem, the protoxylem elements are situated on the inner side (endarch xylem). The phloem is exarch, i.e. the metaphloem elements are adjacent to the xylem, while protophloem is on the outer side of the bundle.
3.2.3.2. The secondary thickening of the stem
Below the zone of primary tissues, in the majority of dicot stems secondary (lateral) meristems establish, that begin to thicken the stem via producing secondary tissues. The secondary conductive tissue elements are formed by the vascular cambium (‘cambial ring’), while the phellogen (cork cambium) produces the periderm that substitutes the decaying epidermis that is unable to follow the surface expansion of the thickening stem.
If the stem has vascular bundles in the stele, the procambium, producing their conductive elements, also forms bundles in the shoot tip. The meristematic derivatives of the procambium are also present within the vascular bundles, i.e. these are open collateral bundles. Prior to the secondary thickening, the cambial strands of the bundles (fascicular cambium) are connected to each other by dedifferentiated strands of the ray parenchyma (secondary meristematic cells, interfascicular cambium) to establish a continuous meristematic cylinder, the vascular cambium 5.
The meristematic cells of the fascicular cambium always produce secondary vascular tissues (but for some rather thin parenchymatic rays), yet the products of the interfascicular cambial regions are various. Based on the activity of the interfascicular cambium, different types of secondary thickenings are distinguished that are overviewed on Figure 4. The apparently continuous secondary conductive tissue is divided by the radial rays, and in the secondary xylem annul growth rings are present (in the thickened stem of woody plants living among appropriate climatic conditions – see below).
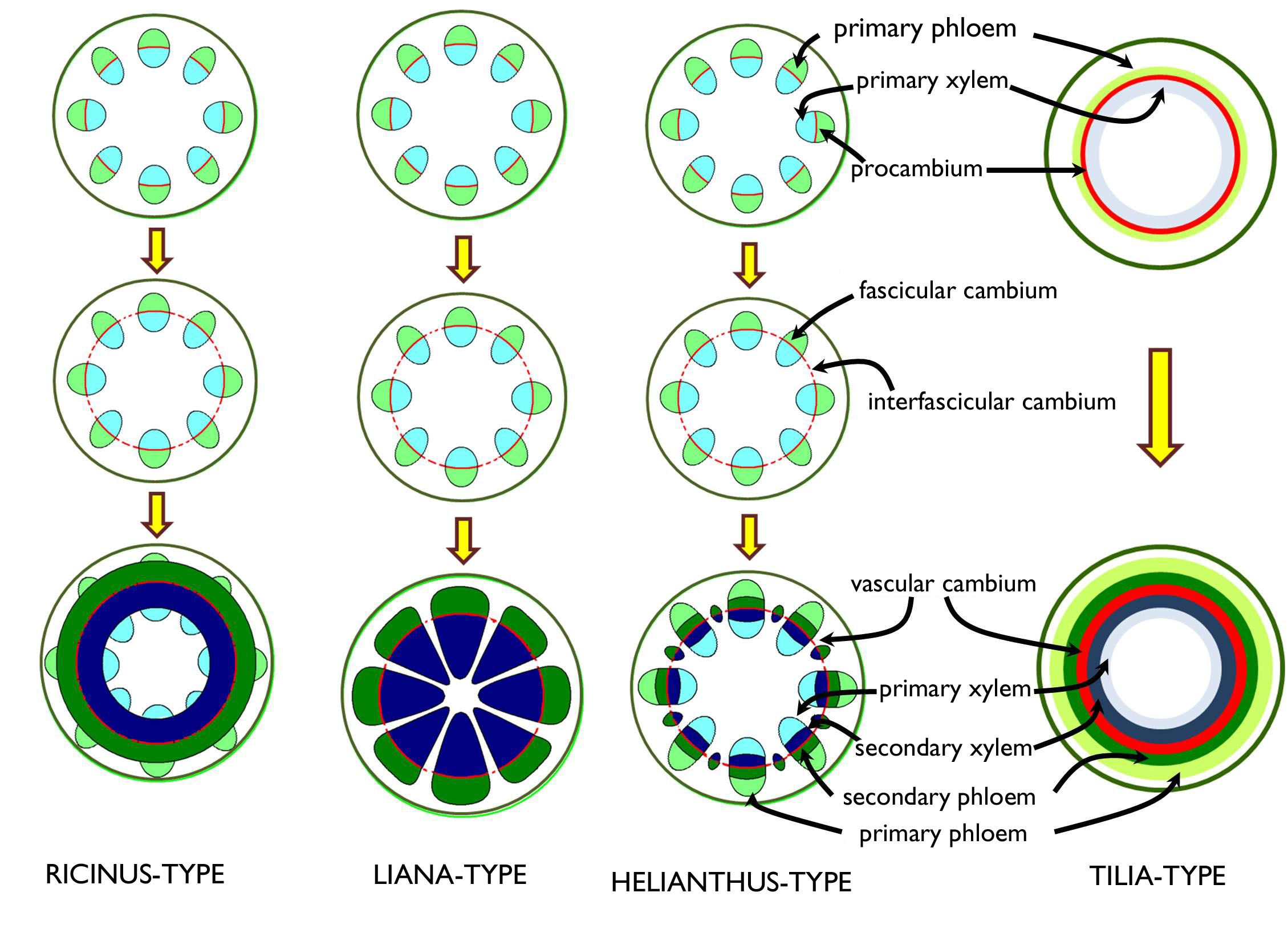
Figure 4. Different types of secondary thickening of the stem.
The woody stem of most gymnosperms and woody angiosperms develops by Tilia-type thickening. In the meristematic zone (shoot tip) of these stems, a continuous ring of procambium is present, thus the primary xylem and primary phloem elements establish as continuous cylinders (i.e. no vascular bundles are observed). In the zone of secondary thickening, the procambium situated between the two types of primary conductive tissues starts to function as a vascular cambium6, and its divisions forms the cells of the secondary xylem and the secondary phloem. This ontogeny results a characteristically concentric arrangement of different tissues: below the primary cortex, the ring of the primary phloem can be observed in cross section, to the inner side of which the layers of the secondary phloem attach; the next tissue layer (‘ring’) is the vascular cambium followed by the ring of the secondary xylem inside. The innermost tissue band is that of the primary xylem that surrounds the pith parenchyma.
3.2.2.3. The structure of the woody stem
The majority of the thickened woody stem is composed of secondary conductive tissue elements (Figure 5). This type of stem is covered by a specialized secondary protective tissue of characteristic anatomy and origin, the bark (rhitidome). The secondary phloem adjoining the bark has the oldest elements in its outermost region, while the youngest cells are adjacent to the vascular cambium. To the interior from the cambial ring the youngest, actually conducting xylem elements are found. Approaching the centre of the stem, subsequently older xylem layers are observed. (Here we stress that one single ring (actually a cylinder) of vascular cambium is present in the wood!) Xylem and phloem layers are connected to each other via radially arranged rows of parenchymatic cells, the rays. These living cells serve the function of accumulation besides their role in radial transport.
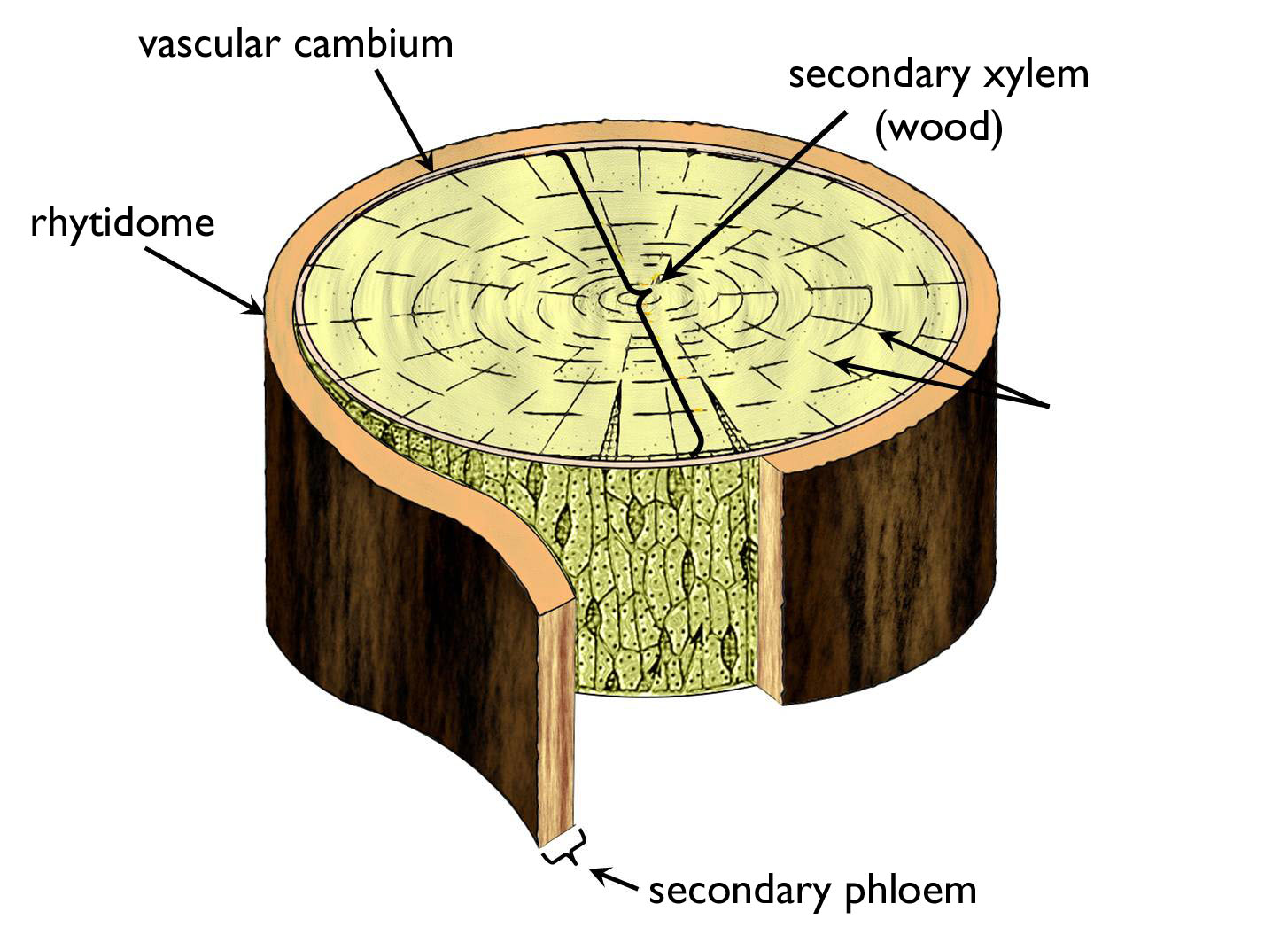
Figure 5. The arrangement of conductive elements within the woody stem.
(Based on Dános)
3.2.2.3.1. The anatomy of the secondary xylem (‘wood’)
Year after year, the secondary xylem of the woody stem grows outwards (centrifugally); the vascular cambium produces the new xylem layers onto the outer surface of the older ones. Consequently, the youngest xylem elements that actually takes part in water and mineral conduction are situated in the outer region of the secondary xylem; the unity of these functioning xylem elements is the sapwood. The older xylem constituents lying to the interior of the secondary xylem do not take part in the transport, these tracheas and tracheids are blocked by the ingrowth of the neighbouring parenchyma cells ( tyloses). In the secondary wall of these xylem cells special secondary metabolites (e.g. phenolic compounds) are deposited so that to prevent the invasion of wood-decaying microbes (chiefly fungi). This inner region of the secondary xylem is called heartwood. In the wood of certain trees (e.g. Fagus, Quercus, Pinus) the deposition of secondary compounds is so intense that the borderline between the sapwood and the heartwood is clearly observable with the naked eye on the cut surface (butt-edge) of the timber 7.
Secondary xylem is principally composed of different vascular (tracheary) elements, parenchyma cells and libriform fibers. The wood of gymnosperms contain only tracheids, parenchymatic cells and fiber-tracheids (libriform fibers and tracheas are absent), thus the microscopic view of these woods is rather homogenous (homoxyl wood)8. In contrast, the wood of angiosperms is composed of much variable tissue elements (tracheids, tracheas, libriform fibers, xylem parenchyma cells and sometimes even fiber-tracheids), thus its anatomical view is much more complex (heteroxyl wood).
Parenchyma cells of the secondary xylem are arranged in two, perpendicular systems: in radial rows comprising the rays (ray parenchyma) and in a longitudinal arrangement attached to the tracheary elements (axial parenchyma).
On the grounds of the lignin content of the secondary wall of xylem elements softwoods and hardwoods are distinguished from each other. The lignin content of softwoods is rather low (below 40%). These species (e.g. Salix, Populus, Pinus, Acer negundo) are of rapid growth and thus more brittle (e.g. more vulnerable to windfall). They are chiefly applied in paper industry. In contrast, hardwoods of high lignin content (e.g. Fagus, Quercus, Taxus, Prunus avium) are quite durable and their timber is utilised as valuable raw material for furniture or building industry. These species can be planted more safely as ornamentals in parks or along roadways 9.
Several plants living among certain climates do not grow continuously: the vegetation periods are interrupted by intervals of dormancy. For instance, in the temperate zone cambial activity correlates with the weather condition (chiefly the temperature) of the seasons. This periodic activity results the establishment of growth rings within the wood10. In spring, following the melting of the soil a high amount of water should be transported through the body of the budding plant from the root toward the growing foliage. In this period thin walled tracheary elements (tracheas, tracheids) of large diameter are formed and the proportion of supportive elements (fibers, fiber-tracheids) is low. This stripe of xylem is quite light in colour and is called springwood (or earlywood) (Figure 8 ). During late summer and in autumn, the rate of transport slows down, and thus narrower vasal elements of thicker cell wall are produced and their proportion is low to the fibers and fiber-tracheids. This stripe is the summerwood (latewood). In winter the cambial activity ceases (or slows down), so the edge surface between the late wood of the previous year and the springwood of the next year is quite distinct: this makes an obvious borderline between the two annual rings. Consequently, the age of the wood can be determined by counting EITHER the dark (summer wood) OR the light rings (spring wood) observed on the butt-edge. The thickness of the annual ring depends on the weather or other environmental conditions of the respective year. For example, in wet years the growth rings are thicker than among harsh conditions (drought, serious pest invasion) 11. Actually, the pattern of the annual rings preserves the ecological history of the tree.
Tracheas can be found in several different arrangements within the angiosperm wood (Figure 6). In diffuse-porous woods, large tracheas are present in both the earlywood and the latewood (e.g. Acer, Aesculus, Fagus, Magnolia, Tilia). On the meantime, in ring-porous wood the large tracheas are found (almost) exclusively in the springwood (e.g. Fraxinus, Catalpa, Morus, Quercus, Robinia, Ulmus).
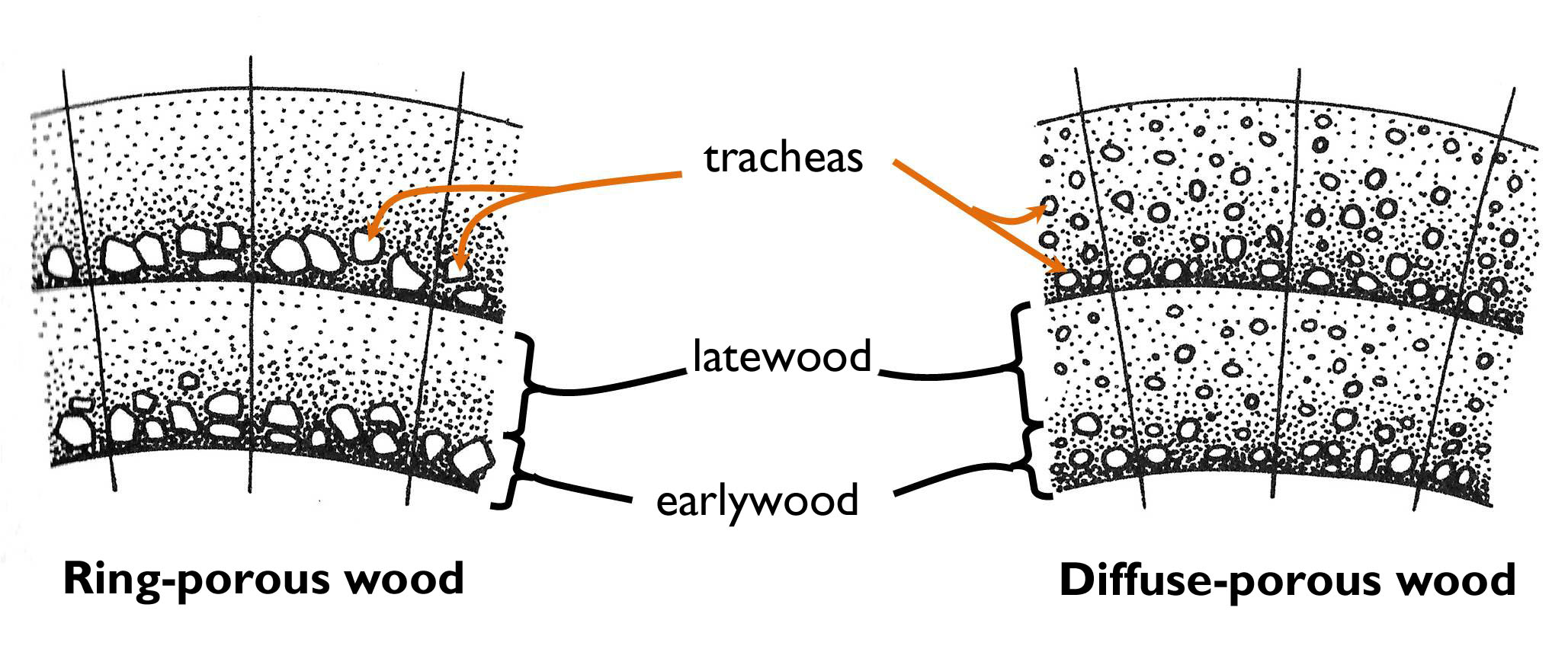
Figure 6. The structure of the wood in a ring-porous and a diffuse-porous tree.
(Based on Jacob et al.)
3.2.2.2.2. The anatomy of the secondary phloem
Similarly to the secondary xylem, the youngest secondary phloem elements are also adjacent to the vascular cambium. However, here this means that the innermost layers are the youngest ones, while the oldest elements face toward the bark of the tree. Thus the differentiation of the secondary phloem is centripetal.
The structure of the secondary phloem is usually storied, like that of the wood. Layers of thick-walled fibers alternate with rows of sieve elements of thinner cell walls. Nevertheless, the periodicity of the ontogeny of these layers does not necessarily follow an annual cycle. Naturally, rays beginning in the xylem are also present in the secondary phloem, yet they broaden towards the perimeter of the stem, forming funnel-shaped structures in cross section (pith rays).
3.2.2.2.3. The rhytidome
The epidermis of young stems – similarly to that of the roots – is unable to expand during the process of secondary thickening. The role of the decaying epidermis is taken over by the periderm. The phellogen producing this protective tissue elaborates via the dedifferentiation of either the epidermis or the parenchymatic cells of the outer cortex12. Even the thickened stem regions of the present year are covered by a periderm, the morphology and structure of which is characteristic of the species.
During the further thickening of the stem, even the periderm ruptures or cracks, sometimes wears off. Then inner cortical parenchyma cells dedifferentiate to form the cork cambium that produces new layers of the periderm. In some species (e.g. apricot (Armeniaca) or apple (Malus)) the elder parts of the woody stem are covered by such a periderm. However, after some time all the cortical cells are transformed into phellogen and the periderm becomes adjacent to the phloem. From this time up, the outermost parenchymatic cells of the phloem form the cork cambium, and the periderm produced by the phellogen of such origin contains the remnants of dead phloem elements (sieve cells, sieve tubes or phloem fibers). This rather thick, modified protective tissue containing phloem elements is the rhytidome (bark). Owing to the subsequent expansion of the stem, the rhytidome also splits up or furrows to form a pattern being characteristic of the tree species. On the meantime, further layers of the secondary phloem turn into phellogen. (This is one reason why the number of the strata of a layered secondary phloem does not correlate with the age of the tree.)
Depending on the taxonomical position of the tree, the bark may contain crystals or other secondary metabolites or colouring compounds. The deposition of such materials results the typical appearance and texture of the rhytidome. For instance, the secondary walls of the cells in the bark of Quercus suber (cork oak) contain a high amount of suberin. The scaled bark of this species is the ‘cork’.
3.2.3. Leaf
3.2.3.1. The evolutionary origin of the leaf: microphyll and megaphyll
Leaf has evolved in several lineages of plants, as a result of parallel evolution. For the first time, it appeared among the ferns. However, the anatomy of the photosynthesising leaves of different taxa might be quite different. Two basic types are distinguished: the microphyll and the megaphyll (Fig. 7). Microphylls are characteristic of the species of the phylum Lycopodiophyta and those of the class Equisetopsida (horsetails) within the phylum Monilophyta. Microphylls are mere superficial projections of the stem (enations or emergences) with vascular bundles, which originate as simple side-branches of the bundles of the stem. The vasculation of the megaphyll appearing first among the ferns (in the phylum Monilophyta), connects rather differently to the vascular system of the stem. From the vascular cylinder of the shoot axis one or more bundles depart toward the leaf (leaf traces) and thus a gap remains within the vascular tissues of the stele (leaf gap). As a consequence of the elaboration of several leaf traces, the conductive tissue system forming a complete cylinder in the ancient vascular plants appeared to be split into a set of vascular bundles. This type of stele is called eustele. Thus, during the course of evolution, the appearance of the megaphyll led to the development of the stele composing of vascular bundles. Naturally, elaboration of leaf traces (and leaf gaps) does not cease the longitudinal transport of the stem. Conductive tissue elements running beside the leaf traces join to each other above the leaf gap, i.e. the vascular system of the stele is not composed of linear tubes but actually it is a system of anastomosing vascular bundles. Beside the ferns, gymnosperms and angiosperms have megaphylls, as well.
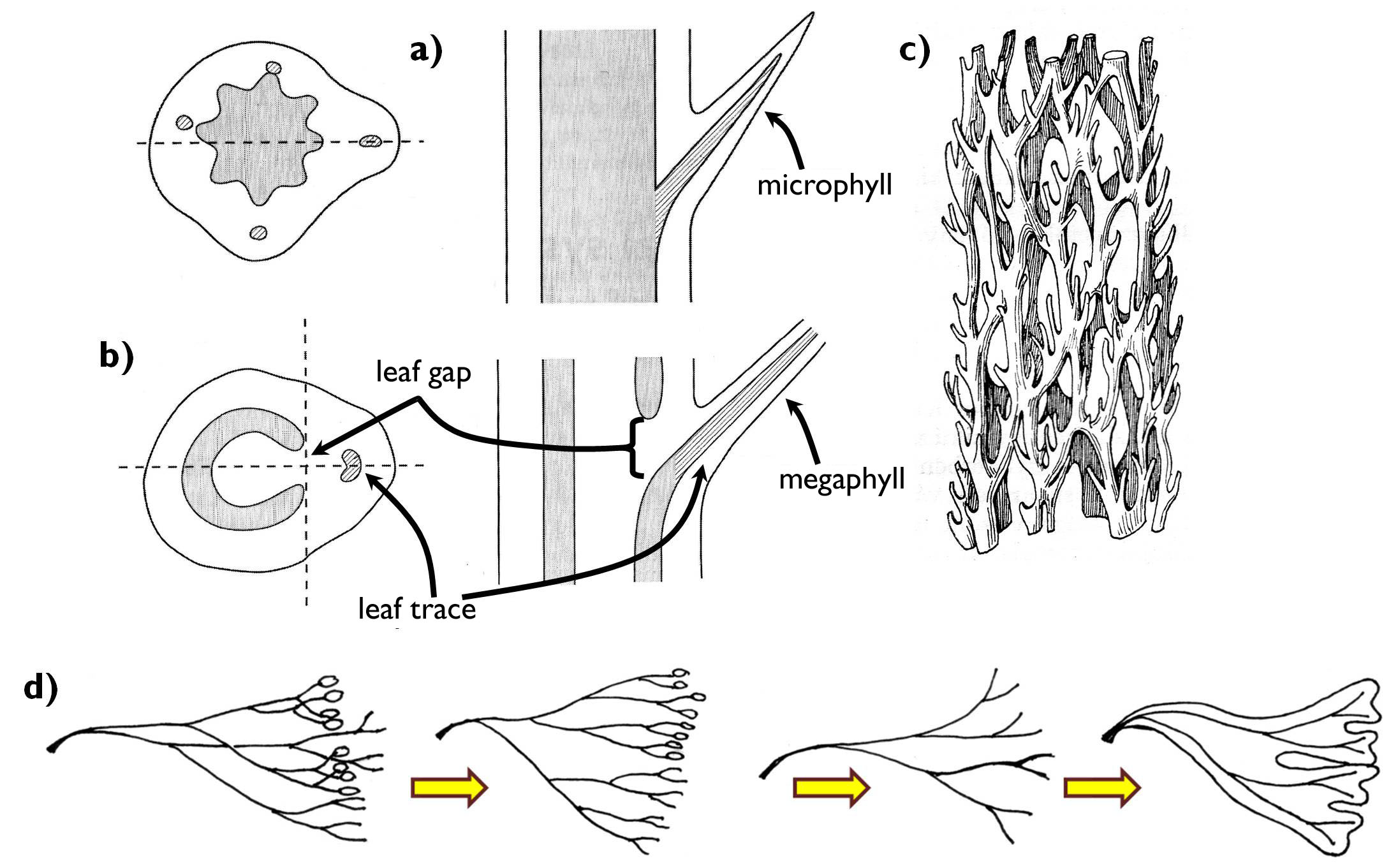
Figure 7. Microphyll and megaphyll. a) connection between the microphyll and the stem;
b) connection between the megaphyll and the stem; c) vascular system of the stem in plants with megaphylls; d) the evolutionary origin of the megaphyll (according to the telome theory).
(Based on: a-b: Beck, c: Kárpáti, d: Podani)
4.2.3.2. The ontogeny of the leaf
As it was mentioned previously, in the shoot the primordia of lateral organs (including those of the leaves) appear already in the zone of primary meristems of the shoot tip. Though leaf primordia are radial in symmetry at the beginning, later they become flattened and their growth becomes determined.
The general process of leaf growth and leaf differentiation may considerably differ among the taxonomic groups. For instance, the growth of the fern leaves is indeterminate for a remarkably long time: while the bottommost region of the leaf has a well-developed lamina, at the tip, undifferentiated meristematic cells continuously produce new tissue elements. Should this tip region get damaged, no complete leaf could develop, thus the meristematic cells should be protected. For this purpose, the differentiated part of the leaf curls up around the tip, and forms a structure resembling the bishop’s crook, called fiddlehead.
Similarly, indeterminate is the growth of the grass leaves (Poaceae). However, in these leaves dividing cells are not on the tip but at the bottom of the leaf. This meristematic zone situated right between the lamina and the top of the leaf sheath continuously produces new tissues. This way of differentiation explains why the grazed or mown leaves of the grasses are still capable of growing 13.
Several leaves of woody plants develop in autumn, right before leaf abscission, and remain in an embryonic stage closed in the buds during the dormant period in the winter. However, notable is the fact that almost all cells are born during bud differentiation, thus no further cell division is required following the budding. Consequently, leaf expansion during the process is the result of mere water uptake, which is an admirably faster process than leaf growth based on cell divisions.
3.2.4.3. Leaf anatomy
The leaf is attached to the stem via the leaf base. This is the region where leaf traces enter the petiole from the stem (in case of sessile leaves, directly into the lamina)14. The petiole of monocots (Liliopsida) contains several leaf traces, while that of eudicots (Rosopsida) has a single or few bundles embedded in ground tissues. The anatomy of the petiole resembles that of the stem, except that all bundles are oriented in the same position, with their xylem facing upwards (i.e. towards the stem, called adaxial) and the phloem facing downwards (i.e. to the lower side or abaxial)15. Petiole is always stiffened by supportive tissues (sclerenchymatic fibers attached to the bundles or layers of collenchyma beneath the epidermis). If the petiole is green in colour, it naturally contains chlorenchyma, as well.
Vascular bundles entering the lamina (leaf veins) produce side-branches in a system determining the type of venation. With the aim of the finest veins, vascular bundles entwine all the lamina. Of course, not all leaf cell has an own end-vein, rather the blind ends of the finest bundles provide nutrients to a small group of the mesophyll (and takes up the photoassimilates from these cells). These smallest functional units of the leaf are called areole s.
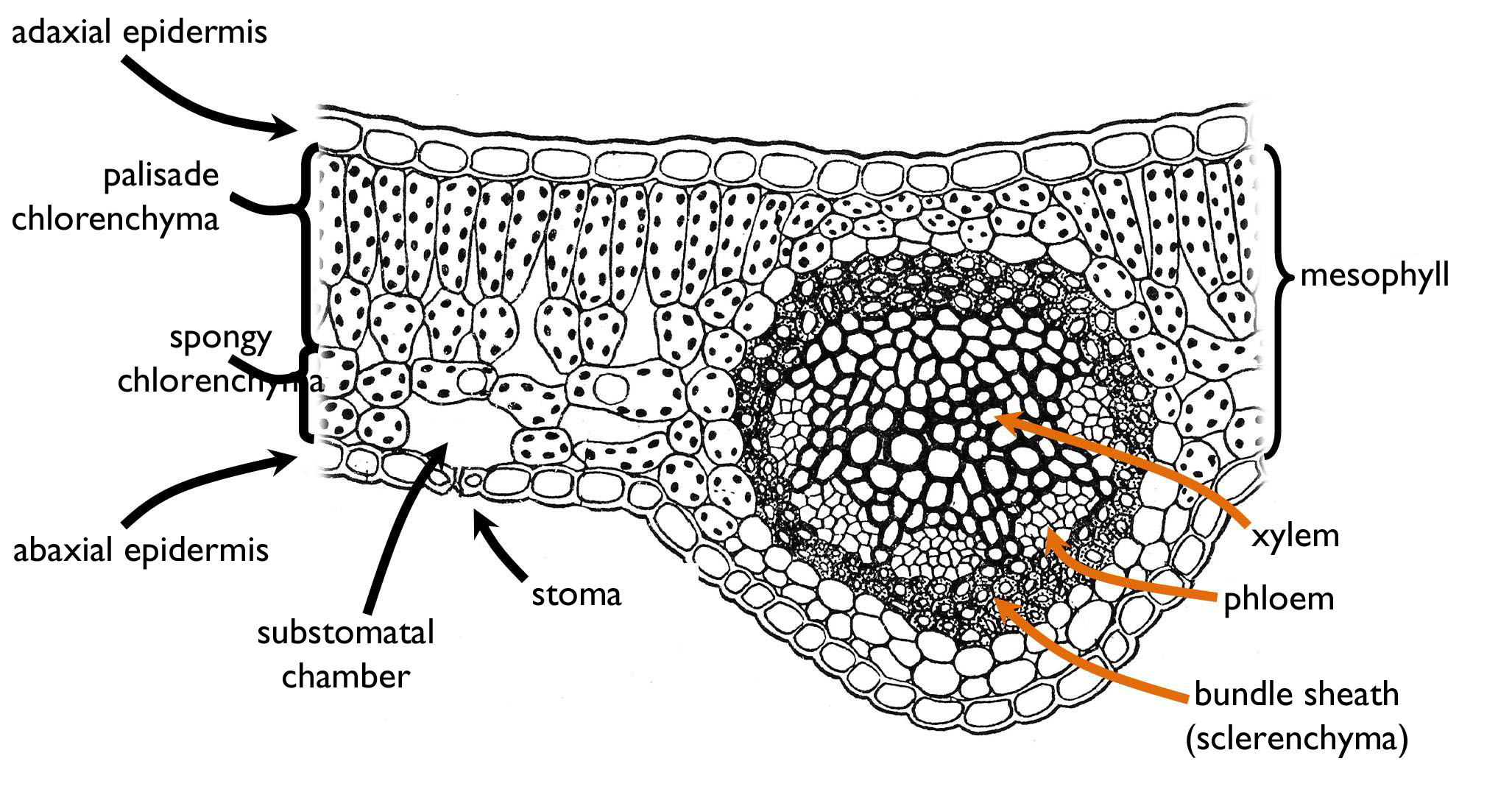
Figure 8. The anatomy of a mesomorphic, dorsiventral and bifacial leaf.
(Based on Sárkány and Szalai)
In cross section, the main tissue region of the leaf, observable between the adaxial and the abaxial epidermis, is the mesophyll ( Figure 8). In leaves of plants living among balanced environmental conditions (mesomorphic leaf) the majority of the stomata are situated on the abaxial side (hypostomatous leaf). The main part of the mesophyll is composed of chlorenchyma that has two different types of cells. In the upper region, photosynthetic cells are elongated perpendicularly to the surface and are packed densely – this is the palisade chlorenchyma. To the contrary, the abaxial side of the mesophyll is composed of irregularly shaped chlorenchyma cells and the system of wide intercellular cavities serving the gas exchange of the cells (spongy chlorenchyma). The intercellular system has a large cavity below the stomatal pore called substomatal chamber that opens directly to the outer world. In the zone of transition between the palisade and the spongy mesophyll, lobed but elongated cells are present. These cells are attached to several palisade cells on the adaxial side and one cell of the spongy chlorenchyma toward the abaxial side. On the cross section, leaf veins are observed as vascular bundles. These bundles are compound, collateral (sometimes bicollateral) bundles with their xylem part facing the adaxial epidermis. Since the growth of most leaves is determinate, their bundles are closed. However, in evergreen leaves – due to their prolonged development – the cambium of open bundles increases the amount of vascular tissue during the ontogeny. Supportive elements of the leaves are principally the sclerenchymatic fibers and the layers of hypodermal collenchyma adjoining the framework of leaf veins.
Like other parts of the shoot, leaves may possess several further tissue types. Secretory elements may be present both in the epidermis (as glands) and in the mesophyll. In the leaves of species containing latex (those of the families Euphorbiaceae, Apocynaceae, Campanulaceae, Papaveraceae) laticifers occur, while resin ducts are frequent in the needles and scale leaves of gymnosperms. Within the chlorenchyma of some species (Laurus nobilis,Hypericum perforatum) oil cavities can be found. Idioblasts filled with different crystal forms are also frequent either in the epidermis (e.g. Ficus) or in the mesophyll (e.g. Malus). In extremely stiff leaves (like the natant leaves floating on water surface – in details see below!) scattered sclereids might be present within the mesophyll.
3.2.4.4. Histological types of the leaf
Foliage leaves can be sorted into different histological categories based on either epidermis anatomy or mesophyll structure (Figure 9.). In both cases, the background of this classification is the relationship between the respective tissue of the adaxial and the abaxial side of the leaf. Identification of the leaf sides is supported by the orientation of the bundles: the xylem determines the adaxial, while the phloem the abaxial surface.
If the epidermis of the adaxial differs from that of the abaxial surface (chiefly due to the different density of the stomata), the leaf is bifacial. This is the most frequent type, because this structure minimises the water loss via transpiration and maximises light exploitation at the same time. Equifacial leaves have identical adaxial and abaxial epidermises. Such an anatomy develops if equal light intensity reaches the two sides of the leaf. Among such conditions, unequal sorting of epidermal structures could not reduce the warming up of the leaf neither the rate of transpiration. For instance, equifacial are the leaves of the genera Viscum, Loranthus or Pinus. Similar is the appearance, yet different is the ontogeny of unifacial leaves. These leaves also have identical epidermis on their total surface, yet have only one side from the developmental viewpoint, because the adaxial surface disappears during the ontogeny. Unifacial leaves might be the results of two different developmental processes (Figure 9). Cylindrical unifacial leaves develop, if the – originally bifacial – leaf curls up toward the adaxial side, then it closes into a hollow cylinder. During the process, the adaxial epidermis is closed within the tube, and later it vanishes. The histological structure of these leaves is rather similar to that of the stem, since the bundles are arranged in circles. Cylindric unifacial leaf is characteristic of the onions (e.g. Allium cepa). The unifacial leaf of irises (Iridaceae) is called ensiform. The lowermost part of these leaves is V-shaped in cross section with a regular bifacial epidermis structure (like an opened book). However, at some distance from the leaf base the leaf closes (like a closing book) and the two halves of the abaxial epidermis are pressed to each other. Later this epidermis reduces, disappears and the mesophyll becomes continuous. In ensiform unifacial leaves, the vascular bundles are arranged in two rows in the lower parts, yet toward the tip they form a single row. However, since the neighbouring bundles derive from different halves of the leaf, they are oriented differently: the xylems of certain bundles face toward one of the leaf surfaces, yet those of the others face to the other side. (That is why we cannot identify the adaxial and abaxial sides on the grounds of the cross section.) Sometimes the bundles of the leaf halves attach to each other and so they form bicollateral bundles in the upper part of the ensiform leaf.
The chlorenchyma in the mesophyll of most leaves is similar to that described in the previous part on leaf anatomy (i.e. palisade chlorenchyma is at the adaxial, spongy chlorenchyma at the abaxial side). Light intensity reaching the surface of these dorsiventral leaves is higher at the adaxial side. This is the reason why densely packed palisade cells of better light utilisation occur at this side. In species with leaves clinging closely onto the stem, the light enters into the leaf from the abaxial side, so the structure of the mesophyll changes: the palisade chlorenchyma is at the abaxial and the spongy chlorenchyma is at the adaxial side of the mesophyll (inverse dorsiventral leaf). The common feature of the previous two types is that different types of chlorenchyma are found beneath the two epidermises (heterolateral leaves). In isolateral leaves, chlorenhcmyas of similar type are present under the adaxial and abaxial epidermis. The two sides of these leaves are usually exposed to similar light intensity. Nevertheless, isolateral leaves may have different types of chlorenchyma in the mesophyll. Below the epidermises of heterogeneous isolateral leav es palisade chlorenchyma is observed, and the interior part of the mesophyll is composed of spongy chlorenchyma (or of isodiametric but more-or-less densely packed chlorenchyma cells). On the other hand, the mesophyll of homogeneous isolateral leaves is unifromly composed of round chlorenchyma cells with few intercellular spaces (e.g. Iris, Viscum).
Of course, the two classification system is not independent from each other. Most dorsiventral leaves are bifacial from the aspect of their epidermis anatomy (e.g. Nicotiana, Fagus, Nerium, Nymphaea), while the majority of equifacial leaves is isolateral (e.g. Galanthus, Viscum, Sedum) just like all unifacial leaves. Nevertheless, some bifacial leaves have homogeneous (isolateral) mesophyll (e.g. Tradescantia, Stipa, Festuca).
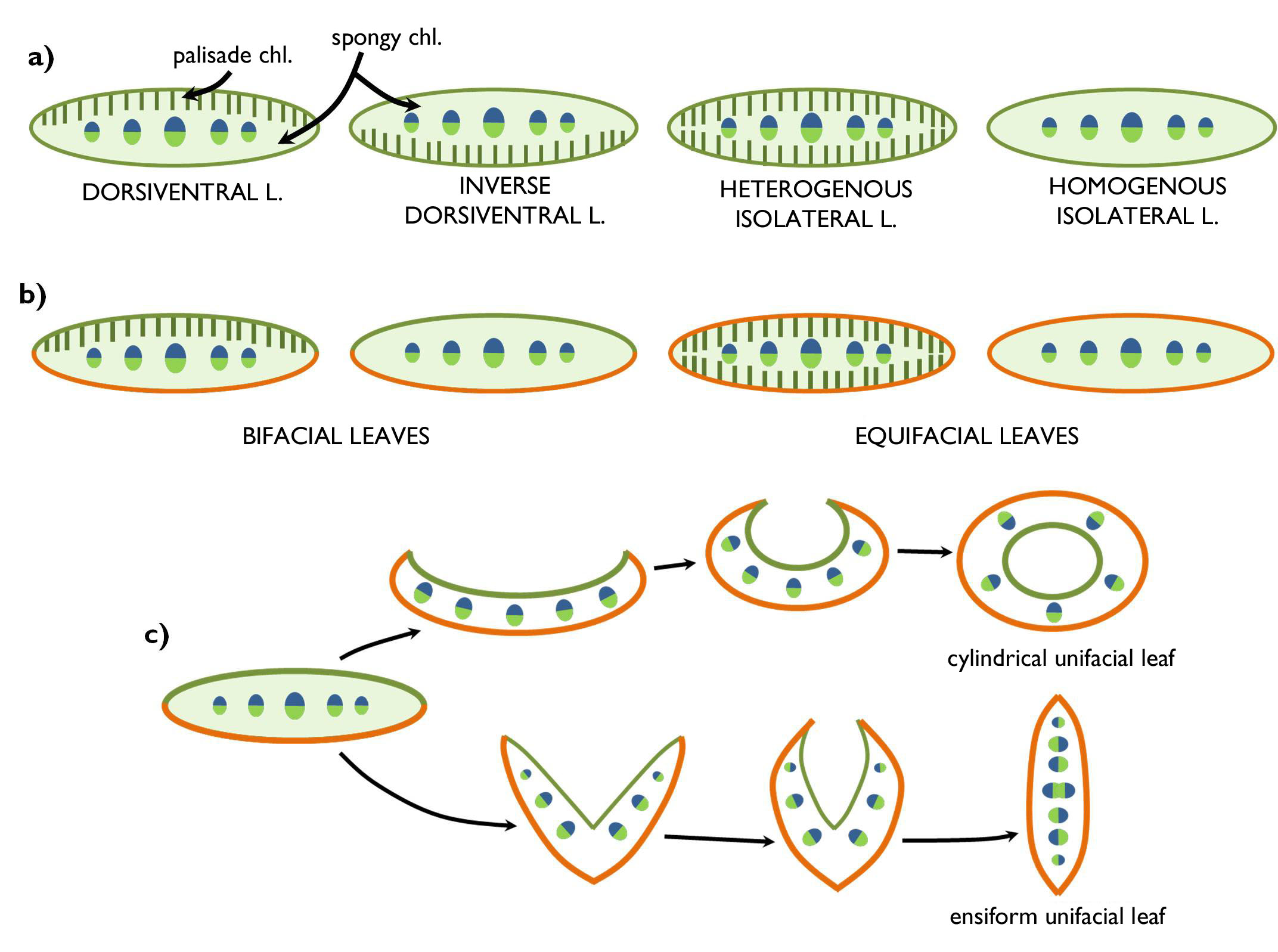
Figure 9. Leaf types concerning the anatomy of the mesophyll (a)
and the epidermis (b,c).
3.2.5. Ecological adaptation of the shoot
The anatomical structure of the shoot may change due to the differing ecological conditions. These ecological adaptations (being the results of evolutionary processes) may serve two different goals: to make the utilisation of environmental sources as effective as possible, or to avoid or decrease the impacts of environmental stresses.
For plant metabolism (photosynthesis) the most important environmental factor is light. The majority of plants use light as efficiently as possible. A certain method to reach this aim is turning their leaves always perpendicular to the direction of the light rays (euphotometric leaves). To the contrary, in habitats with excess irradiation light may be a stress factor for the plants. Surplus light heats up the leaves what increases the rate of transpiration and thus the risk of desiccation. At the same time, light stress has physiological components, as well: in photosynthesising cells high amount of oxygen radicals (reactive oxygen species) are produced that oxidise and destroy the organelles, or may even cause the death of the cell (oxidative stress). Consequently, plants of such sunny areas (e.g. rocky grasslands, sand steppes) try to avoid light stress by developing small leaves or pan-photometric leaves that turn their edge to the direction of the solar rays (i.e. parallel to the sunlight) so as to minimise the amount of the captured radiation16.
If a plant has a remarkably large (and dense) canopy, leaves situated at different parts of the canopy have to cope with unequal light conditions: on the well-lit surface of the foliage sun leaves are present, while in the dark depth of the canopy so-called shade leaves are found. The extension of sun leaves exceeds that of the shade leaves, their mesophyll is thicker and the proportion of palisade parenchyma (that is more active in photosynthesis) admirably higher in these leaves (Figure 10). To the contrary, in the small, thin (often long-petioled) shade leaves the proportion of spongy chlorenchyma surpasses that of the palisade layers.
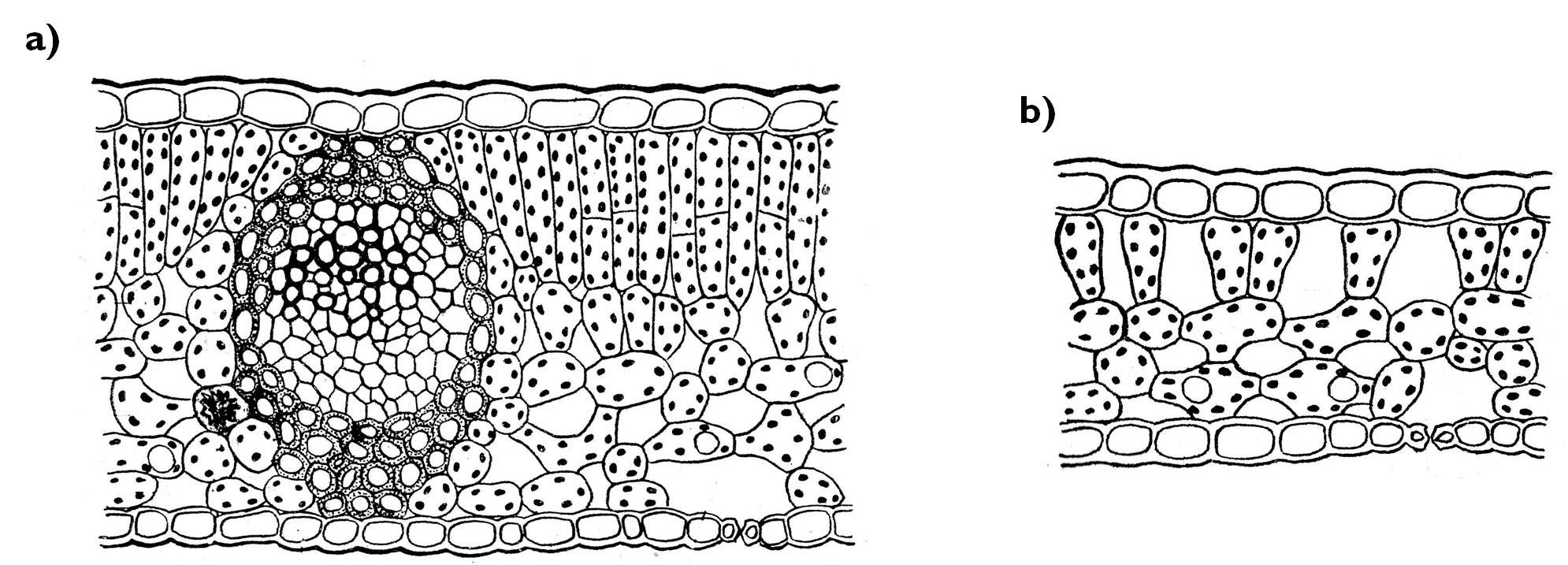
Figure 10. Anatomy of a sun leaf (a) and a shade leaf (b) in cross section.
(Based on Sárkány and Szalai)
An extremely serious stress for the plant is the intense UV radiation – occurring chiefly in the highlands. Plants protect themselves against the harmful effects of this kind of stress by dwarfing (i.e. forming very short internodes). Thus, among such condition vegetation is composed mainly of cushion-like plants called chamaephytes. UV-stress is avoided by shading of the overlapping leaves. The shoot is often densely covered by epidermal hairs composed of dead cells filled with air. These trichomes are light in colour, and the white surface of the plant reflects a considerable proportion of the radiation, and so prevents the damage of inner tissues.
The most important environmental factor that requires specialised morphological and anatomical adaptations from the plant is water. Although water uptake is principally influenced by the root, water content of the body is also controlled by the regulated water delivery of the leaves (and sometimes also the stem). As a reference, we will compare the shoot characteristics of plants living among extreme humid conditions to the features of species inhabiting sites of balanced water content (mesophytes, mesomorphic organs) (Figure 11). Plants of moist habitats (e.g. the herb layer of closed woodlands, marshes, swamps) are called hygrophytes (their leaves are hygromorphic). The leaves of these species of humid areas are rather thin, with a poorly structured mesophyll. Palisade chlorenchyma is hard to distinguish from the spongy layers, since the whole mesophyll contains vast intercellular spaces. Since water can always be taken up from the soil, the turgidity of the cells is more-or-less constant, what is enough to support the organs. Owing to this fact, few supportive elements (fibers, sclereids) are present in the shoot; nevertheless, these plants easily wither (e.g. Impatiens, Corydalis). Their leaves are amphistomatous, i.e. stomata occur on both sides of the leaves in a similar density. The guard cells are coplanar with other epidermal cells. If the humidity of the air around the leaves is close to the saturation point (e.g. in the undergrowth of rainforests), transpiration may become inhibited. However, due to the osmotic suction force of the root water is continuously taken up from the soil and root pressure drives the xylem solution through the whole body, what results the exudation of small droplets through the epidermis of the shoot. This process, called guttation, is mediated by modified stomata called hydatodes. These anatomical structures are also present characteristically on hygromorphic leaves (e.g. Zantedeschia). A further feature of these leaves, often covered by a watery film, is that their tip is long and pointed (‘drip tip’) so that to drain off the water from the surface. This can reduce the risk of leaf rot and the colonisation of pathogens.
Plants of humid habitats are the hydrophytes (waterplants). Their hydromorphic leaves are of two main types: submersed leaves floating within the water (e.g. Potamogeton, Myriophyllum) or natant leaves floating on the water surface (e.g. Nymphaea, Nuphar, Hydrocharis)17. Both leaves are capable of adsorbing water and minerals right from the water surrounding them, thus their vascular bundles are quite small. Especially the xylem part of the bundles is reduced. In addition to nutrients, submerse leaf takes up oxygen and carbon-dioxide also by diffusion through the epidermis, thus no stomata are present on the leaf surface. Consequently, these leaves have an extended surface, i.e. they are deeply lobed, yet considerably thin with reduced (or often missing) mesophyll. The role of the absent chlorenchyma is taken over by the epidermis, so – as an exceptional case – the epidermal cells of these hydrophytes contain chloroplasts. Naturally, no cuticle covers the leaves. Since these buoyant leaves are supported by the upward pressure of the water, almost no supportive tissue is present. In contrast, natant leaves are spread out by the sclerenchymatic fibers and hypodermal collenchyma strands running along the leaf veins. The mesophyll of these leaves is quite thick composing of a multi-layered palisade chlorenchyma and an extended spongy chlorenchyma with vast intercellular cavities (aerenchyma). The aerenchyma filled with air floats the natant leaf on the water surface. This spongy tissue is supported by scattered sclereids. Stomata are present – obviously – only in the upper (abaxial) epidermis (epistomatous leaf). This surface is also covered by a thick, water-repellent cuticle that prevents the leaf from soaking.
If the amount of available water is limited in the soil, the plant (xerophyte) has two possible strategies: it may either minimise the rate of transpiration or it accumulates as much water as possible. The first goal might be achieved by reduction of the total area of transpiring organs. Leaves may be reduced into scales (Calluna, Tamarix) or leaf spines, what chiefly coincides with the elaboration of phylloclades, cladodes or cladophylls (e.g. Asparagus, Ruscus). In some genera (e.g. Euphorbia), the leaves of the plants wither in the dry season and the function of photosynthesis is fulfilled by the stem right till the beginning of the next rainy season. Leaves bearing anatomical characteristics serving the reduction of transpiration are called xeromorphic leaves. The surface of these leaves is generally minimal, and they contain a high amount of supportive elements so that to prevent the collapse of the leaf during water loss. Epidermal transpiration (i.e. water-loss through the epidermal cells other than stomata) is reduced by a thick cuticle. Several anatomical modifications serve the elaboration of a water saturated environment around the stomata. One of these is the presence of dense trichomes on the surface. In this case, vapour is trapped among the hairs, what reduces the water delivery via diffusion. Trichomes of the epidermis are often composed of dead cells filled with air, which results a rather light, whitish surface decreasing the amount of light entering into the leaf. The less energy is absorbed by the leaf, the lower is its temperature and thus lower is the rate of transpiration. Stomata are usually sunk beneath the level of other epidermal cells. The epidermis of certain species (e.g. Nerium oleander) invaginates into the mesophyll forming stomatal crypts. Stomata opens into this chamber filled with hairs, where the air becomes quickly saturated with water vapour. The epidermis of the leaves of several grass species (Poaceae) contains bulliform cells. These cells of unequal cell wall thickening are capable of changing their shape in response to water loss of the tissue, resulting the curling up of the leaf into a hollow cylinder (for details see chapter 2!). In this case, the air space within the ‘leaf tube’ is saturated with vapour, which reduces the rate of transpiration via the stomata opening into this cavity.
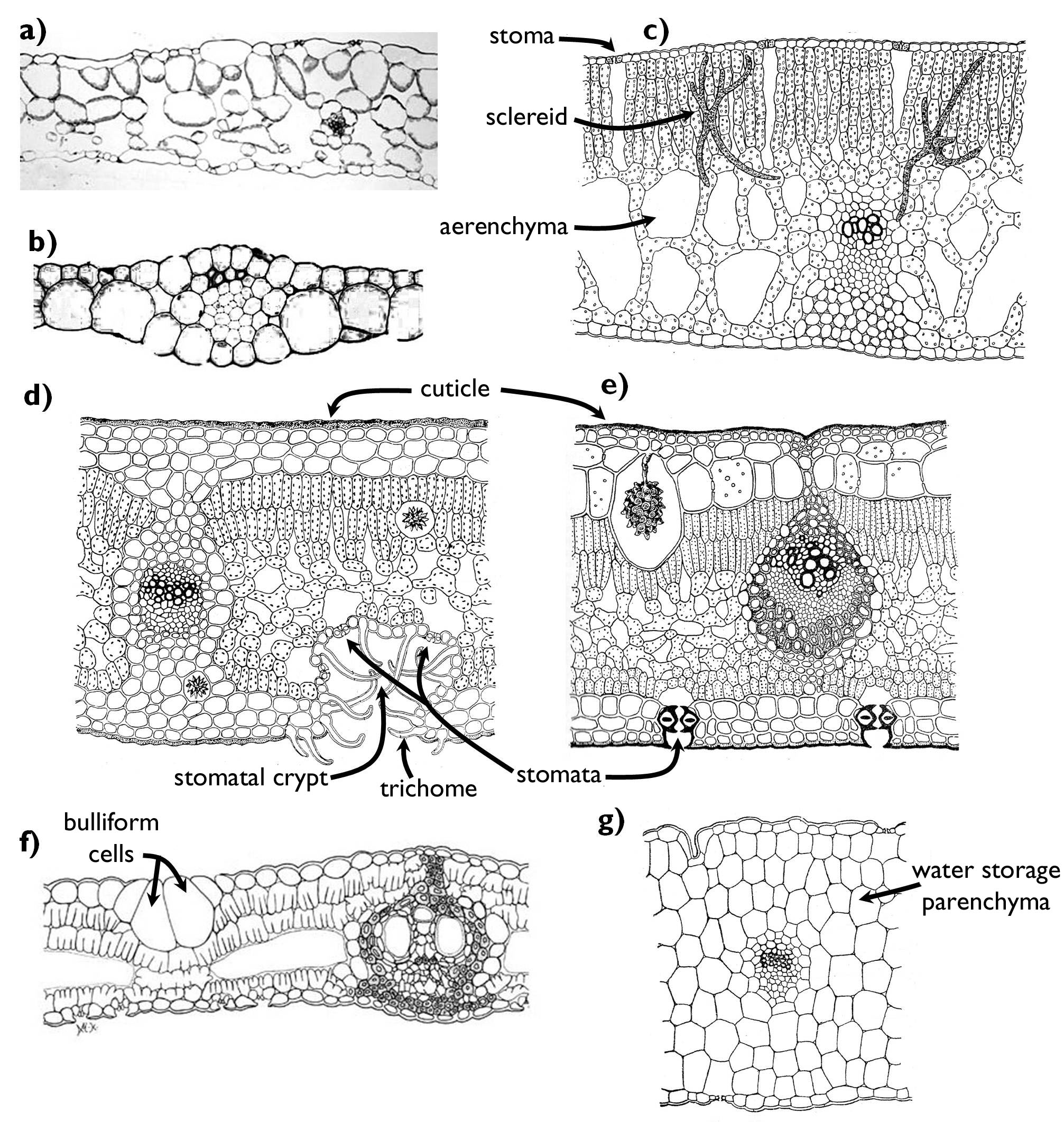
Figure 11. Anatomy of leaves adapted to environments of different water content.
a) hygromorphic leaf (Impatiens); b) hydromorphic-submers leaf (Elodea);
c) hydromorphic-natant leaf (Nuphar); d-f) xeromorphic leaves (d: Nerium, e: Ficus, f: Phyllostachis);
g) succulent leaf (Sedum).
(a, b and f: based on internet-derived figures: a, www.cas.miamioh.edu, b: esu.edu,
f: www.eeob.iastate.edu/research/bamboo/characters/anatomy.html; c-e, g: based on Sárkány and Szalai)
For the purpose of water accumulation, xerophytes develop succulent organs. Water storage tissues may be in the roots (Chlorophytum), the stem (in the families Euphorbiaceae and Cactaceae or the genus Adenium), in the leaf blade (Sedum, Sempervivum) or the petiole ( Zamioculcas, Rheum). The most important characteristic of succulent leaves is the water storage tissue composed of cells with a large vacuole filled with hygrophilous mucilage. Naturally, succulent leaves also have anatomical features that reduce the intensity of transpiration, e.g. waxy epidermis or sunken stomata. As a result of water loss minimisation and water accumulation maximisation, succulent leaves have a low surface to volume ratio, thus they are round or cylindrical.
Drought is not the only abiotic factor that results the development of xeromorphic features. Each environmental effect that hinders water uptake (or increases water-loss) leads to the establishment of the above mentioned characteristics (due to physiological drought). Intense solar radiation and heath stress accelerate transpiration, while the frost hampers the uptake of the frozen groundwater. In case of salt stress (e.g. in halophytes) the increased concentration of the soil solution hinders the water absorption of the root. Consequently, plants of the boreal forests or the tundra, as well as those living in coastal habitats are all characterised by xeromorphic morphology and/or anatomy 18.
An adaptive physiological characteristic of the species living in sunny and hot habitats is that during the day they close their stomata so that to minimise the water loss. However, this results low carbon-dioxide concentration within the leaf tissues right at the time when light reaction can proceed. This problem was solved by developing alternative ways of photosynthesis during the course of evolution. The general way of carbon-dioxide fixation is the so-called C3-photosynthesis. During this process, CO2 is fixed in the form of a compound of three carbon atoms (glycerate-3-phosphate) that is transformed into glucose in the dark reaction (Calvin-cycle) with the application of ATP and NADPH produced by the light reaction. In the photosynthetic cells of C3-plants light and dark reactions happen at the same time, within the chloroplasts of the same cell. In several plants of hot and dry habitats an alternative route, C4-photosynthesis takes place instead. In these species CO2-fixation and -transformation into glucose occurs in two different cell types spatially separated. The first step happens in the mesophyll cells, where CO2 is incorporated into an intermediary compound of four carbon atoms (malate, oxal-acetate or serine). This four-carbon molecule is then transported into the bundle-sheath cells surrounding the vascular bundles of the leaf. Here CO2 is released, and utilised to generate glucose via the C3-pathway (i.e. in the Calvin-cycle). The advantage of C4-photosynthesis is that the concentration of CO 2 can be increased within the bundle sheath cells to such rate, which enables effective glucose production even when the stomata are closed.
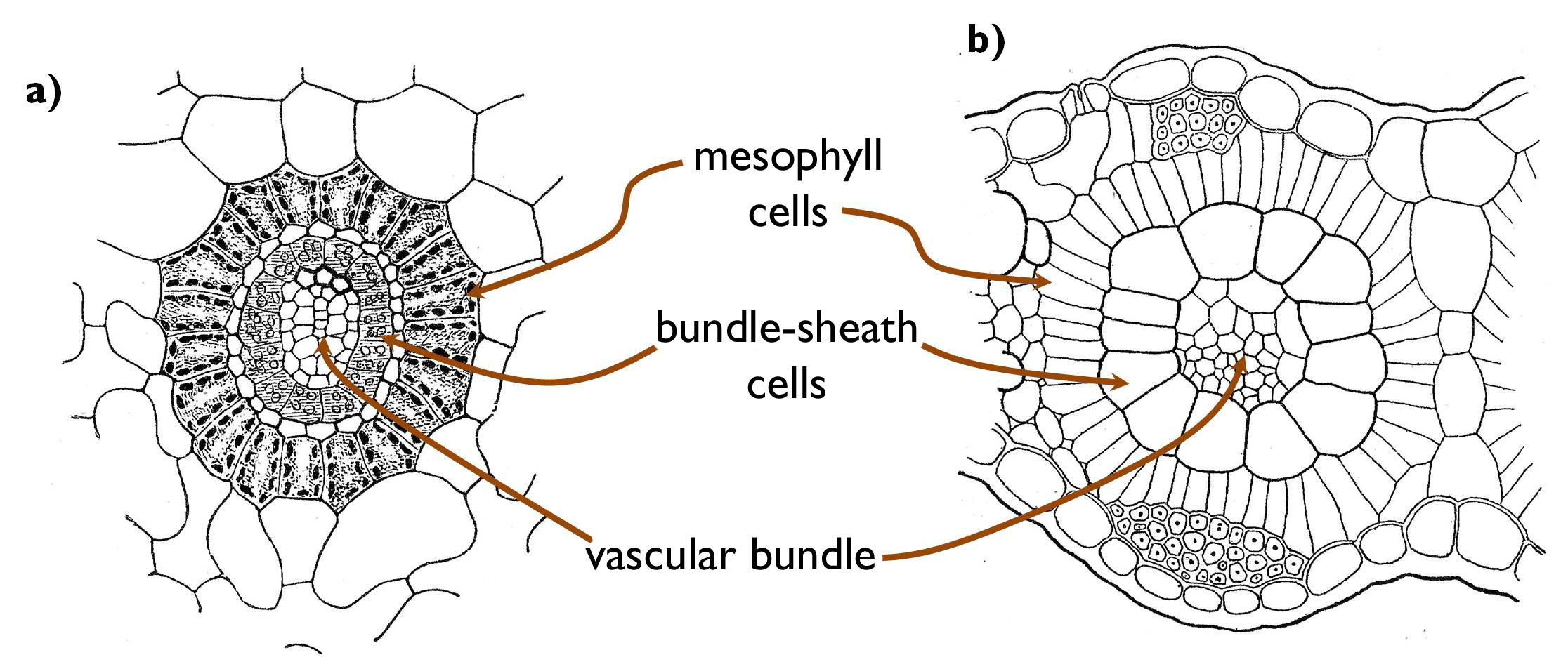
Figure 12. Kranz-anatomy. (a: Cyperus papyrus, Panicum miliaceum)
(Based on Habelrandt)
The physiological adaptation of C4-photosynthesis requires anatomical alterations, as well. Bundle sheath cells are arranged in concentric, radial rows around the vascular bundles, and outside from these occur the rings of the mesophyll cells (Kranz anatomy) (Figure 12 ). Besides, in the majority of the C4-species, ultrastructural changes also occur. The chloroplasts of the mesophyll cells have grana but lack starch grains, because no glucose production happens here. (The presence of grana indicates that light reaction occurs here, which produces ATP and NADPH required for the primary fixation of the CO2 into the C4-compound). To the contrary, from the chloroplasts of the bundle sheath cells grana are missing (since no light reaction takes place here), yet starch grains are present storing the produced glucose. The phenomenon described is called the plastid dimorphism of the C4 plants.
References
Beck CB (2010): An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century. 2. kiad. Cambridge University Press, Cambridge,
Biebl R, Germ H (1967): Praktikum der Pflanzenanatomie. 2. ed., Springer-Verlag, Wien-New York.
Braune W, Leman A, Taubert H (1967): Pflanzenanatomisches Praktikum. VEB Gustav Fischer Verlag, Jena.
Cutler DF, Botha T, Stevenson DW(2008): Plant Anatomy: An Applied Approach. Wiley-Blackwell, Oxford.
Erős-Honti Zs (2012): A kertészeti növények alaktana. Egyetemi jegyzet. Budapesti Corvinus Egyetem Kertészettudományi Kar, Bp.
Evert RF, Eichhorn SE (szerk.) (2006): Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body – Their Structure, Function, and Development, 3. kiad., Wiley-Interscience, New Jersey.
Gyurján I (1996): Növényszervezettan. Egyetemi jegyzet. ELTE Eötvös K., Bp.
Haberlandt G (1924): Physiologische Pflanzenanatomie. Verlag von Wilhelm Engelmann, Leipzig.
Jacob F, Jäger EI, Ohmann E (1985): Botanikai kompendium. Natura, Bp.
Kárpáti Z, Görgényi L, Terpó A (1968): Kertészeti növénytan I. Növényszervezettan. Mezőgazdasági Kiadó, Bp.
Mauseth JD (2008): Plant Anatomy. The Blackburn Press, New Jersey.
Sárkány S, Haraszty Á (1995): Növényszervezettan biológia-kémia, biológia ill. pszichológia-biológia szakos hallgatók részére. Egységes jegyzet. Nemzeti Tankvk., Bp.
Sárkány S, Szalai I (1966): Növénytani praktikum I. Növényszervezettani gyakorlatok. 3. kiad., Tankvk., Bp.
Wilson K, White DJB (1986): The Anatomy of Wood: its Diversity and Variability. Stobart & Son Ltd., London.
1 The structure of the root tip meristems may be different in other taxonomic groups. For instance, in the family Poaceae root cap and rhizodermis is produced by two separate meristems, the calyptrogen and the dermatogen, respectively.
2 The vascular cambium of the root is a complex meristem,,concerning its origin. Since the cells of the pericycle retain their meristematic activity, these regions of the cambium (neighbouring the xylem bundles) are regarded as primary meristem. However, the cambial region lying between the xylem and phloem bundles are composed of dedifferentiated parenchymatic cells, i.e. these are considered as secondary meristem.
3 Consequently, the cork cambium of the root is also a ’complex’ meristem (similarly to the vascular cambium) consisting of meristematic strands of primary and secondary origin.
4 The process of nitrogen fixation cease in the presence of oxygen (due to the enzyme destruction caused by this gas), thus within the nodules oxygen is bound by a special molecule called leg-haemoglobine. This compound is quite similar to the haemochrome of the vertebrates, haemoglobin. Both metalloproteids contain ferric ion and both of them is red. Owing to the presence of leg-haemoglobin, the functioning nodules are pink in colour.
5 Similarly to the vascular cambium and the phellogen of the root, vascular cambium of the stems bearing vascular bundles is also of complex origin. The strands of fascicular cambium is considered as primary meristems, while the interconnecting cells of interfascicular cambium should be regarded as secondary meristems.
6 So actually, a primary meristem serves the function of secondary thickening.
7 Quite frequently, the similar phenolic compounds are deposited in the wood around a wound or an infection point of pathogenic microbes, what results a conspicuous patch on the butt-edge that looks similar to the heartwood. This coloration is called false heartwood or false kernel.
8 The homogenous wood of the gymnosperm families Pinaceae and Cupressaceae contain resin ducts supported by sclerenchymatic fibers and lined with secretory epithel cells.
9 In plant anatomical literature other interpretation of the terms ‘hardwood’ and ‘softwood’ can also be read. In these works ‘softwood’ refers to the wood of gymnosperm lacking fibers, while angiosperm trees containing also fibers have ‘hardwood’. Consequently, this usage is independent from the physical properties of the wood.
10 In contrast to the cold and temperate climatic zones, where the dormancy is caused by the low temperature and the frosts of the winter, in the zone of steppes and on the savannah, the division of the cambial cells is ceased by the arid season.
11 Since the trees of a certain area grow among rather similar ecological conditions, the pattern of their annual rings is also alike. Moreover, by comparing this pattern of a cut down (or fallen) tree with that observed on a tree of known age, we can determine the date of cutting (or fall) of the previous one. This principle is the background of the scientific dating method called dendrochronology (tree-ring dating).
12 Interestingly, in grapevine (Vitis vinifera) deeper cell layers of the cortex, neighbouring the phloem develop into phellogen.
13 Interestingly, the differentiation of the tissues is also unique in the grass leaves. In case of the majority of the megaphylls, the direction of differentiation is acropetal, i.e. from the leaf base toward the tip, while in the leaves of Poaceae the first differentiated tissues occur at the tip of the leaf while the youngest (and least differentiated) elements are around the leaf base (basipetal differentiation).
14 In several monocots, leaf traces form a separate cylinder within the cortex (i.e. outside the stele!).
15 This orientation is obvious seeing that of the bundles of the stem. If we bend the leaf onto the stem, the adaxial side of the leaf faces towards the centre of the stem, thus the bundles of the leaf run parallel to those of the stem. Consequently, since xylem of the stem faces toward the centre, no change in bundle orientation occurs in the leaf.
16 The leaves of some plants cannot respond to the direction of incoming rays by turning their blades. These are called aphotometric leaves.
17 In case of some hydrophytes both leaf types occur on the same plant (e.g. Trapa natans, Ranunculus aquatilis). This phenomenon is a special (ecological) type of heterophylly.
18 Of course, the mentioned stress factors require unique adaptations from the plants, as well. For example, the epidermis of salt tolerant species has salt glands capable of excreting the excess salt. Plants of frosty habitats have to cope with the damaging effect of the ice crystals formed within their body.

