Plant reproduction
Author: Zsolt Erős-Honti
4.1. Sexual and asexual reproduction in plants
Similarly to other organisms, the process of plant reproduction may serve two different goals: to increase the number of individuals or to facilitate genetic recombination. Forms of reproduction that primarily aim at the increase of the offspring number are called asexual reproduction, while the processes enhancing genetic diversity of the population are the ways of sexual reproduction (Table 1).
During asexual reproduction the genetic material characteristically do not change (i.e. no genetic recombination occurs), thus the offspring (here clones) are exact genetic copies of the parent, i.e. they are identical from the genetic point of view. No gametes are produced in the process and meiosis (cell division serving recombination) does not happen, either. Several types of asexual reproduction are known: via spores generated by mitosis (mitospores), by the fragmentation of the vegetative body (e.g. pieces of a moss) or by modified vegetative organs (e.g. stolons, bulbs). Since this type of reproduction does not enhance genetic variability, it does not serve the adaptation of the plant population to the changing environment. Consequently, asexual reproduction is advantageous among more-or-less constant environmental conditions.
The aim of sexual reproduction is changing the genetic information by recombination, i.e. providing genetic variability for the population. Among multricellular organisms, this form of reproduction includes the events of meiosis, gamete production (gametogenesis) and gamete fusion (fertilisation). Organs of sexual reproduction are called generative organs (e.g. gametangia, flower, seed, and fruit – depending on the taxonomical position of the plant). The number of offspring is far less than the amount of those produced by asexual reproduction, yet their genetic material differs both from each other and also from the parents. In natural populations, the principal goal of this type of reproduction is the adaptation to the changing environment.
Table 1. Overview of asexual and sexual reproduction.
|
Asexual reproduction |
Sexual reproduction |
|
|
Principal aim |
increasing the number |
enhancing genetic diversity |
|
Recombination |
absent |
occurs |
|
Genetic information of the offspring |
identical, also with |
differing, also from that |
|
Most important events |
sequence of mitoses |
meiosis, gametogenesis, fertilisation |
|
Reproductive organs |
(modified) vegetative organs |
generative organs |
|
Environmental conditions |
more-or-less constant |
rapidly changing |
4.2. General features of plant reproduction
4.2.1. Reproductive cells of the plants
Two types of reproductive cells have significant roles in the life cycle of plants: gametes and spores. Gametes are haploid cells fusing each other in the process of fertilisation resulting the first diploid cell of the life cycle, the zygote. Gametes may be motile zoogametes (planogametes) or immobile aplanogametes. In case of terrestrial vascular plants, male and female gametes are conspicuously different. Spermatocytes are the smaller ones produced in much higher amount, while oocytes are large cells, always incapable of moving and parents produce them in a lesser number. In certain plants gametes are born in gametangia called antheridium (male gametangium) and archegonium (female gametangium).
Spore s are thick-walled reproductive cells adapted for the survival of unfavourable periods and serving also the dispersal of the species. In early evolved groups of plants (among algae) spores might be mobile, flagellate zoospores, but motionless aplanospores are more frequent. Although most plant spores are born via meiosis (meiospores), some algae produce spores by mitosis as well (mitospores). The latter ones are the reproductive cells of asexual reproduction. (Here we would like to stress that meiospores should not be confused with the gametes! Gametes are haploid cells produced by mitosis and are fused in the process of fertilisation to form the zygote. To the contrary, meiospores divide by mitoses to establish the body of the gametophyte – see below in details!)
Concerning the appearance and the sex of their spores, two different type of plants are distinguished. Homosporous plants produce morphologically identical spores (homospores) that germinate to form either bisexual or unisexual gametophyte. Homospores and bisexual gametophyte is characteristic of the hepatophytes and the majority of terrestrial ferns, while the homospores of several bryophytes and the horsetails (Equisetopsidea) develop into a bisexual (‘dioecious’) gametophyte. Heterosporous plants produce two, morphologically different types of spores, in two different types of sporangia. The smaller microspores are the male spores, produced in higher number. They germinate into a gametophyte bearing antheridia with spermatocytes. The other spore is larger (megaspore) and born in far lesser amount. Later it establishes the female gametophyte with archegonia and egg cells. Some aquatic ferns and all seed plants have heterosporous life cycle.
4.2.2. General features of the plant life cycle
The life cycle of all plants is the alternation of haploid and diploid phases. At a certain stage of all the cycles, haploid gametes are produced that fuse to form the diploid zygote (fertilisation). Among algal species, several different types of life cycles can be distinguished on the bases of the composition and proportion of diploid and haploid stages. Nevertheless, embryophytes (mosses and vascular plants) have an identical, general type of life cycle called haplodiplontic, heteromorphic cycle.
The general cycle has two main stages, both composed of more than one cells (Figure 1). The diploid (2n) stage is the sporophyte that lasts from the zygote right till the spore mother cells. The haploid (1n) gametophyte starts with the production of spores and the last cells are the gametes. Spores are born via meiosis and develop into the simple prothallium (or protonema in mosses). Later the haploid body give rise to the gametes. Fertilisation (i.e. the production of the diploid zygote) is the event that leads into the phase of the sporophyte. The zygote always divides to form an embryo that differentiates into the diploid body (sporophyte) of the plant. On the sporophyte, diploid spore mother cells are produced that divide by meiosis to start the new gametophyte phase with the spores.
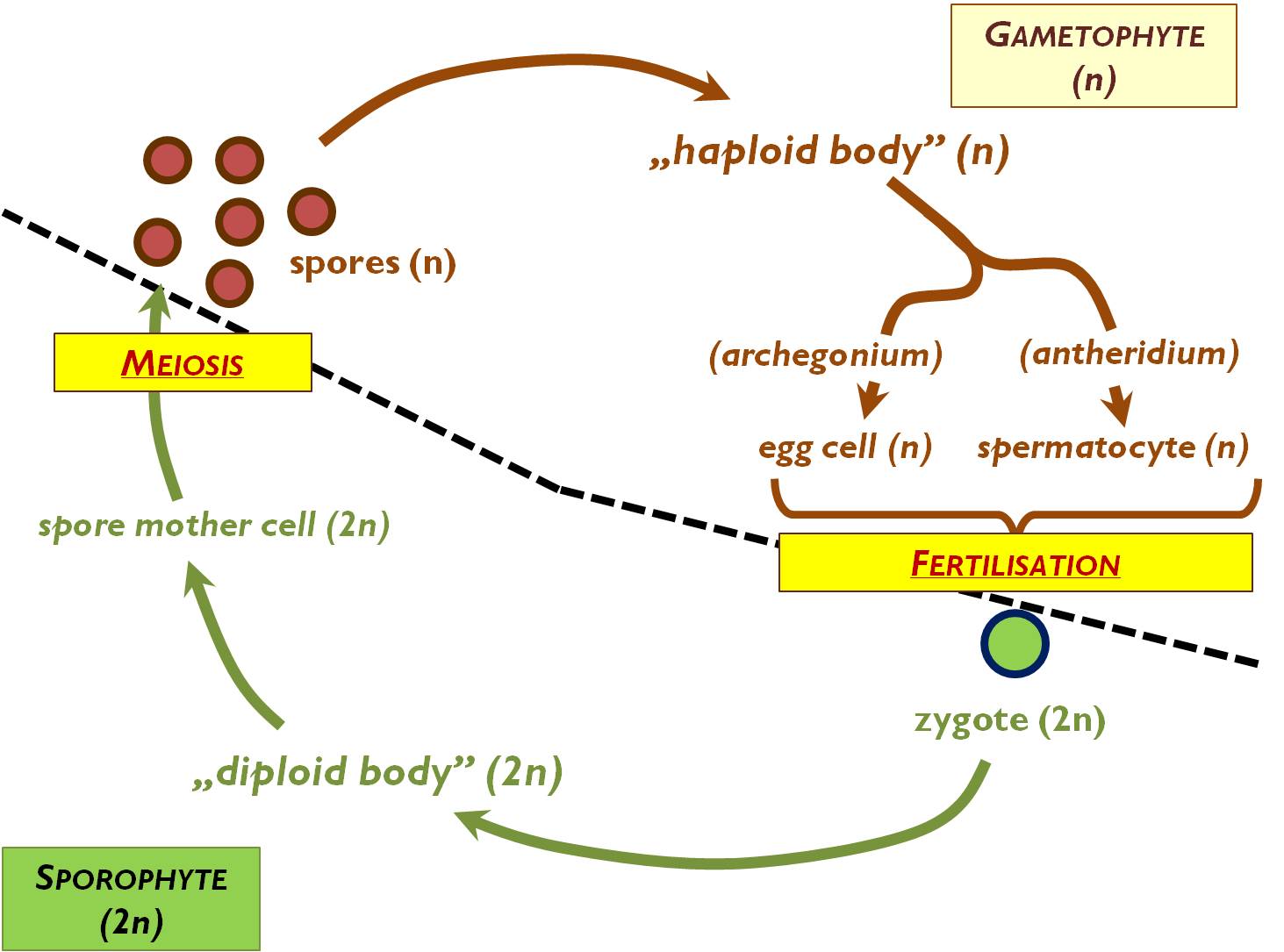
Figure 1. General plant life cycle.
4.3. Asexual reproduction and asexual propagation
The common aim of asexual reproduction and asexual propagation is to produce as many offspring as possible of the same genetic material, which is the result of natural or artificial selection. In horticulture, the natural organs of asexual reproduction are often utilised, i.e. bulbs (Allium sativum, Lilium bulbiferum), plantlets produced on viviparous leaves (Kalanchoe tubiflora, K. daigremontiana), runners (Fragaria × ananassa, Sempervivum tectorum), stolons (Convallaria majalis) or the rooted nodules of creeping stems ( Vinca major), bottom or root sprouts (Salix, Populus), tubers (Solanum tuberosum) or chopped rhizomes (Iris). Additionally, further artificial methods of asexual propagation have also been invented (Figure 2).
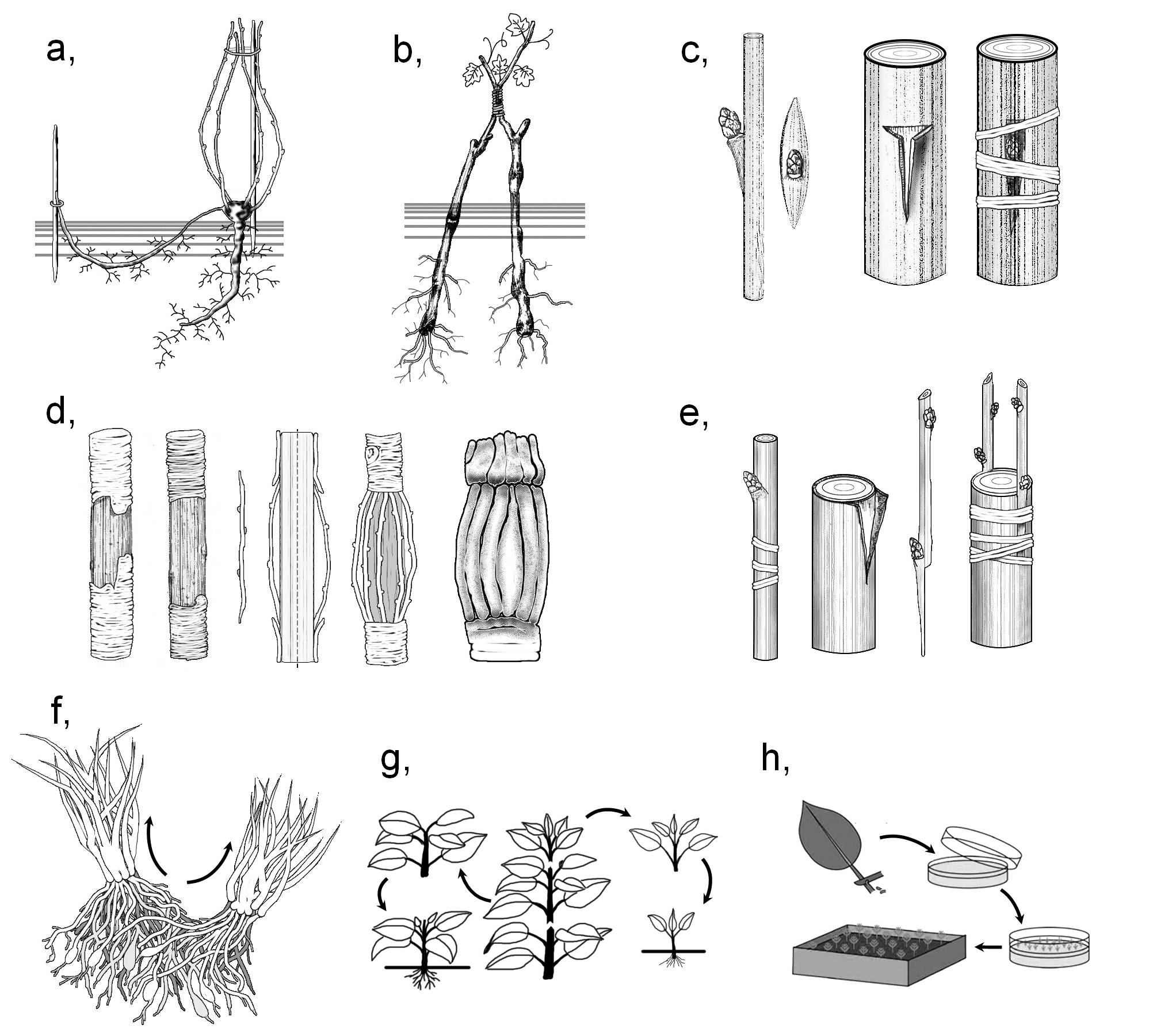
Figure 2. Possible methods of asexual propagation.
a) layering; b) approach grafting; c) bud grafting; d) bridging; e) grafting; f) division;
g) cutting; h) micropropagation.
(Based on : a-e: Kárpáti, f-h: internet-derived figures; f - answers.com, g - pubs.ext.vt.edu, h - bbc.co.uk)
Perennial plants and bushes of dense architecture or those forming polychormones can be broken into individual plantlets with own roots and shoots. This method of propagation is called division. This category includes the separation of sprouts, as well as the cutting up of subterranean modified stems (e.g. stolones or rhizomes). In the method of layering, an aerial shoot of the plant is bent and buried into the soil. On the nodules within the moist rooting medium adventitious roots establish then the buds give rise to new shoots. Following their development, these plantlets can be separated from the mother plant. (Layering is typically the propagation method applied for woody plants. 1)
In the method of cutting (striking) a vegetative organ is separated from the source plant and then it is placed into water or any moist rooting medium (peat, sand or vermiculite) where it produces new roots and shoots. On the cut surface usually wound callus develops, from which adventitious roots originate subsequently. Regeneration of the shoot starts from actually present or adventitious buds. On the bases of the cut organ several types of striking are distinguished: stem cuttings (woody cuttings, semi-woody cuttings or green cuttings), leaf cuttings (applied e.g. forBegonia, Peperomia, Sansevieria), petiole cuttings (for Saintpaulia) or root cuttings (used for Armoracia lapathifolia or Papaver orientale).
Transplantation means the separation of an organ from a source plant (scion) that is inserted into the wound made on the target plant (rootstock). Thus, a selected cultivar of desirable properties (fruit size, taste, flower colour etc.), which is usually quite vulnerable, can be cultivated among unfavourable conditions owing to a resistant rootstock. Consequently, we can propagate the scion in an admirably short time and in high abundance.
Subsequent to the insertion of the scion into the wound, on the cut surface of both plants wound callus develops (following the necrosis of the superficial cells), that produces an intermediary tissue of parenchymatic cells. This partially breaks through the wound surfaces, yet at certain regions it presses these surfaces to each other. This results the elaboration of a distinct borderline between the plants. Later the intermediary tissue penetrates also this borderline, meanwhile its cells dedifferentiates and starts to divide. At the same time, the wound is covered by wound cork toward the outer world so that to defend the healing junction. The next event is the elaboration of parenchymatic strands through the intermediary zone that attach the living tissues of the scion to those of the rootstock. On the meantime, the cambium of the partners starts to penetrate into the intermediary tissue. Finally, the cambium of the scion connects to that of the stock forming a common, continuous cambial zone, which starts to produce the common secondary tissues of the partners. By the joining of the vascular tissues (inosculation) the plants are completely fused together.
Several different techniques of grafting are applied in the horticulture. In case of budding (bud grafting) a single bud (‘eye’) is transplanted, in contrast with shoot grafting when complete shoot portions are propagated. In approach grafting (approach) the transplanted shoot of the scion remains attached to the source plant. During this method, wounds of similar size are made on both plants, wounds are attached to each other and the plants are tightly bound. Scion is separated from the mother plant only following the inosculation. By this technique such sensible organs can also be transplanted, that would otherwise die before the fusion of the tissues. By bridging damaged parts (e.g. rotten branches or trunks) can be replaced. In this case, scions are attached at their both ends to the stock, one end on each side of the damaged region.
The most recent way of asexual propagation is micropropagation (cloning). This technique enables the propagation of those species that cannot be produced by either of the above mentioned methods (e.g. orchids or carnivorous plants). On the meantime, by cloning researchers has the possibility to change the genetic material of the propagated plants. At first, (usually meristematic) tissues are isolated from the source plant and the cells are maintained among in vitro conditions, on artificial media containing hormones. At the beginning, a callus of undifferentiated cells is established, which is later made to differentiate into vegetative organs by changing the proportion of the hormones (chiefly that of auxins and cytokinins) of the substratum. Of course, several calluses can be produced from the cells of a single source plant, and the calluses can also be divided during the process, thus the method indeed serves the function of propagation. (The genetic material of the cells in the callus can be altered by respective molecular biological techniques, yet the description of these is beyond the purpose of the present work.)
4.4. Sexual reproduction
4.4.1. Flower
4.4.1.1. Concept
The flower is a stem of short internodes and determinate growth bearing modified leaves serving the function of sexual reproduction. For the first time, flower evolved among ancient gymnosperms. (However, some researchers accept the term ‘flower’ only for the respective structure of angiosperms.)
4.4.1.2. The structure of the flower
The flower of angiosperms is composed of the modified stem (floral axis) and the modified leaves serving reproductive function (including sporophylls). Floral axis consists of the pedicel and the expanded tip of the pedicel (receptacle) to which the floral leaves are attached. The outer whorls of the flower comprise the perianth (sepals comprising the calyx, petals comprising the corolla or tepals of the perigone), while the inner whorls are the evolutionary descendants of the sporophylls (generative leaves).
Complete flowers possess all the leaves (i.e. the perianth, the stamen and the carpels), otherwise the flower is incomplete. Some anemogamous flowers completely lack the perianth (e.g. Salix, Populus). From the sterile flowers both the stamens and the carpels are missing; their function is chiefly the attraction of the pollinators. Imperfect flowers lack either the stamens or the pistil, in contrast with perfect flowers with both anthers and pistil. Imperfect flowers may be male or female flowers depending on the reproductive leaf present within. In the flowers of certain species (e.g. Fraxinus angustifolia subsp. danubialis) both stamens and pistils are present, yet one type of them is vestigial. These flowers are the functionally imperfect fowers.
Plants with imperfect species may be dioecious or monoecious. In the previous case, both male and female flowers are present on different individuals (e.g. Salicaceae, Urtica dioica, Humulus lupulus, Cannabis sativa). Monoecious plants have both male and female flowers on the same individual (e.g. Fagaceae, Betulaceae, Cucurbitaceae, Zea mays, Begonia sp.).
4.4.1.3. Ontogeny of flowers, and flowering
Seed plants are considered as mature following the development of the first flowers. In case of annual species this event occurs in the same year of germination, while most perennial species produce flower (or compound) buds in the year previous to flowering.
Flowering may be timed variously both during the vegetation period and also within a single day. Woody plants may flower previous to foliation (e.g. Cornus mas, Prunus amygdalus), subsequent to sprouting (Cornus sanguinea, Vitis vinifera) or the two events may be concurrent (Acer platanoides, Prunus persica). Naturally, this phenomenon depends on the composition of the buds of the species. Species flowering prior to foliation always have flower buds containing only the primordia of generative organs. Species of compound buds (i.e. those containing embryonic leaves and flowers as well) flower concurrent to or after foliation. (Obviously, plants with separated flower and foliage buds have the possibility to flower at the same time of foliation if the buds burst out concomitantly – e.g. Prunus persica.)
The time of flowering may be influenced by the lengths of light and dark periods (photoperiodism). Short-day plants (e.g. Chrysanthemum × hortorum, Coffea arabica, Oryza sativa) originating from tropical and subtropical regions flower only under a continuous dark period not shorter than 12 hours (i.e. the light period is up to 12 hours) (Table 2). In the temperate region, these plants flower in mid-summer or even later in the year. In contrast, long-day plants, deriving from the temperate or boreal climatic zone, flower in spring or at the beginning of summer, since they require at least 12-14-hour-long light period (e.g. Pisum sativum, Triticum aestivum, Lactuca sativa). For these species, the lengthening of the days is indicative of the warm-up of the weather, and thus the end of the dormant period2. (The timing of flowering might also be independent from the day length – these are the day-neutral species.)
Table 2. Comparison of short-day and long-day plants.
|
Short-day plants |
Long-day plants |
|
|
Origin |
tropical or subtropical |
temperate or boreal |
|
Flowering in central Europe |
late summer, autumn |
spring, early summer |
|
Light conditions |
continuous dark period at least of |
dark period up to 8-10 (max. 12) hours |
|
examples |
Chrysanthemum × hortorum Coffea arabica Nicotiana tabacum Gossypium hirsutum Oryza sativa Saccharum officinarum Euphorbia pulcherrima Kalanchoe blossfeldiana |
Dianthus caryophyllus Hyoscyamus niger Secale cereale Lolium perenne Trifolium pratense Campanula sp. Pisum sativum Hordeum vulgare Lactuca sativa Triticum aestivum |
4.4.1.4. Anatomy of the flower parts
The anatomy of the floral parts serve as proper evidence of the fact they evolved from modified shoot organs. The structure of the pedicel resembles that of the stem, with a single exception that the pedicel has elaborated supportive tissues (sclerenchymatic bundle sheath, hypodermal collenchymas). The amount of these tissues further increases during fruiting, because this floral part will turn into the fruit pedicel that has to hold even the heaviest fruits.
The anatomy of the members of the perianth is similar to that of the photosynthetic leaves (Figure 3.a.): between the two epidermises a mesophyll of parenchymatic tissue is present, containing collateral closed vascular bundles. The structure of the sepals (or even the outer tepals of some species) resemble most precisely to that of the leaves: their epidermis contains functioning stomata, the mesophyll is composed of chlorenchyma, often with a layer of palisade cells on the abaxial side (yet the structure is mostly homogeneous isolateral). No functioning stomata occur in the epidermis of petals and tepals, however, the surface of these organs is ornamented with papillae. The mesophyll is composed of thin-walled, isodiametric parenchyma cells often containing different pigments determining the colour of the corolla (perigony). (Purplish-reddish petals contain anthocyans, yellow colour is due to betalains or flavonoids, orange and red colours are the results of carotenoid accumulation, green colour is cause by the chlorophylls.) (White perianth does not have any specific pigment. In this case the leaf reflects the total spectrum of the visible light.) Bundles of the perianth are remarkably reduced, composing of few vascular elements.
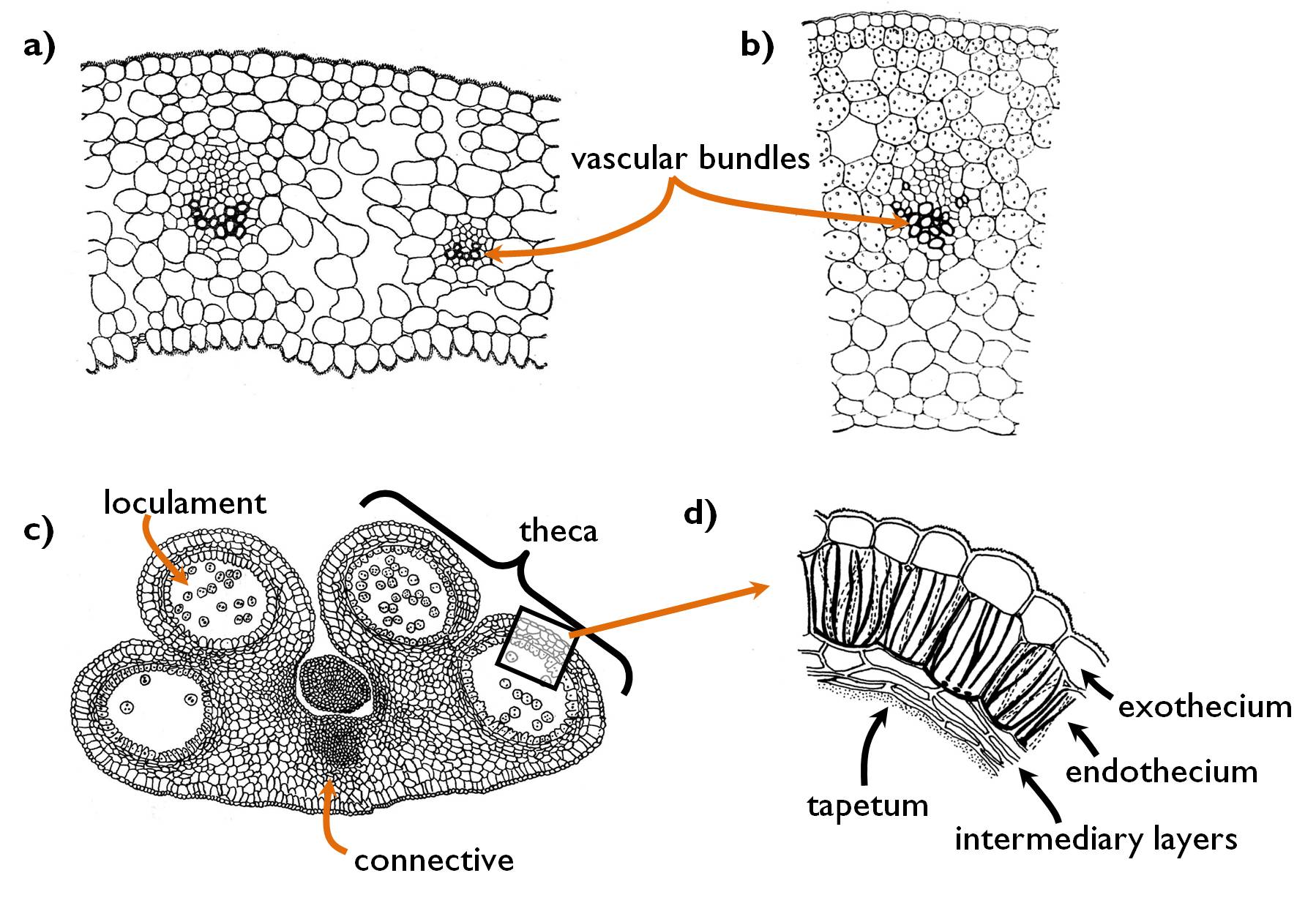
Figure 3. Anatomy of the floral organs. a) structure of the sepalum (Helleborus); b) anatomy of the carpel comprising the wall of the ovary (Tulipa); c) structure of the anther (Lilium); d) layers of the anther wall.
(Based on Sárkány and Szalai)
Androecium is composed of the stamens. All stamen consists of two parts, the filament and the anther (Figure 3. c-d). The filament attaches to the anther in the region of the connective that connects the two halves of the anther (theca) to each other. The single collateral closed bundle of the filament continues within the connective. Each theca contains two loculaments (i.e. microsporangia), thus altogether four loculaments are present in the anther. The wall of the anther consists of four distinct tissue layers. The outermost is the exothecium, which is the single-layer epidermis of the stamen. The next tissue (endothecium) is composed of cells with characteristically reticulate cell wall thickening. Following the development of the pollen grains, the cells of this layer loose water, what results a tension force that dehisces the anther wall. Adjacent to the subepidermal endothecium, intermediary layers occur, that may be absent in some species. The loculament is lined with the innermost tissue layer, the tapetum. Tapetum cells produce the materials of the centrifugally thickening secondary wall of the pollen grains. These compounds are deposited onto the outer surface of the vegetative cell amd comprise the characteristic ornamentation of the pollen grain.
The gynoecium of the angiosperm flower consists of the pistil, which originates as the fusion of the carpels. The uppermost part of the pistil is the stigma, where the pollen grains are captured, the style that elevates the stigma above other floral parts and the ovary containing the ovules. Ovules are attached to a specific region of the interior ovary wall called placenta.
The surface of the stigma is covered by a papillate glandular tissue that secretes sugary mucus trapping the pollen grains 3. The centre of the style is filled with transmitting tissue that leads the pollen tube toward the ovarial chamber. The tube grows within the polysaccharide layers of the cell wall of the stylar tissue. Besides, the cells of the transmitting tissue nourish the tube, as well.
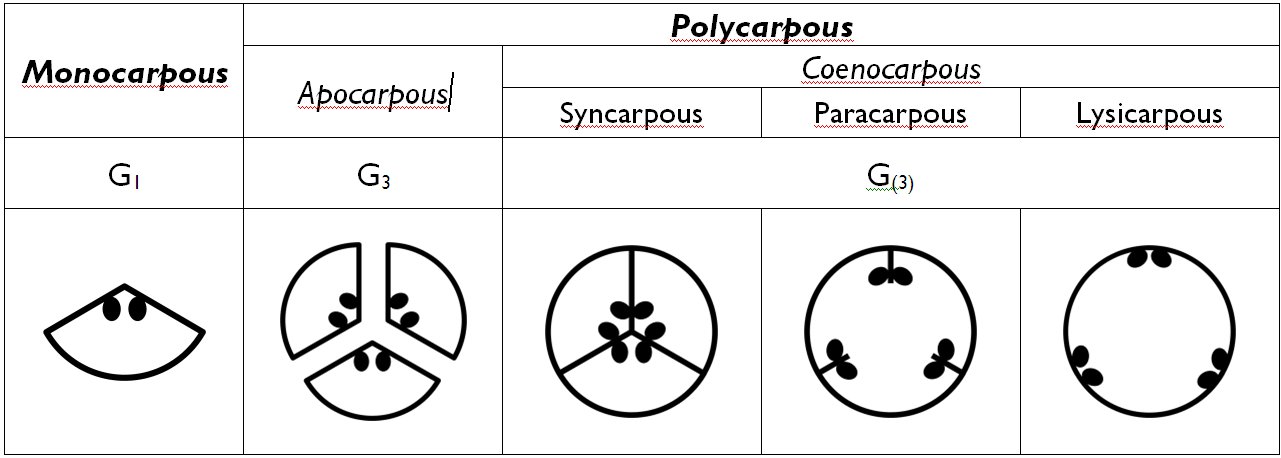
Several different types of ovaries are distinguished on the bases of the number and fusion of the carpels (Table 3). Monocarpous (unicarpellate) gynoecium consists of a single carpel that fused in a cylindrical manner (e.g. Prunus, Berberis, Fabaceae). If more than one carpels comprise the gynoecium, it is called polycarpous. If each carpel forms an individual pistil (i.e. they are unfused) the gynoecium is apocarpous (choricarpous). Should the carpels fuse to from a common pistil, the gynoecium is called coenocarpous. The chamber of the coenocarpous ovary may be completely divided into locules by septa – syncarpous gynoecium. If the septa are partially disappeared, but their remnants project into the single ovarial chamber, the gynoecium is paracarpous. Finally, in some species the coenocarpous ovary has a single, undivided chamber without any sign of previous septa – this is the lysicarpous gynoecium.
Table 3. Overview of different types of the gynoecium.
4.4.2. Microsporogenesis and microgametogenesis in angiosperms
Microsporogenesis of angiosperms begins with the meiotic division of the microspore mother cells (pollen mother cells) happening within the sporogenous tissue of the anther (Figure 4). The products of the division are four microspores surrounded by a common cell wall composed of callose. Thus the four microspores are attached to each other to form a tetrad. (In some species, even mature pollens occur in tetrads within the anther – e.g. in the family Ericaceae.) The cells in the tetrads are nourished by the tapetum cells. Under the callose layers, right the microspores begin to elaborate the cell wall of the latter pollen grain.
During the synthesis of the durable cell wall composing of sporopollenin, the callose is digested, the microspores separate and divide by mitosis to produce the two haploid cells of the angiosperm microgametophyte: the large vegetative (tube) cell and the smaller generative cell. The latter migrates into the cytoplasm of the vegetative cell, thus the wall of the pollen grain is actually the secondary wall of the vegetative cell. The pollens of most species are released in this binucleate stage (binucleate pollen), yet in about one third of the plants (e.g. in the family Poaceae) the generative cell produces the two male gametes (sperm cells) within the anther. In this case the released pollen has three nuclei ( trinucleate pollen). The generative cell of binucleate pollen grains goes through mitosis only in the germinated pollen tube. (Consequently, in these species microgametogenesis is finished in the pistil.)
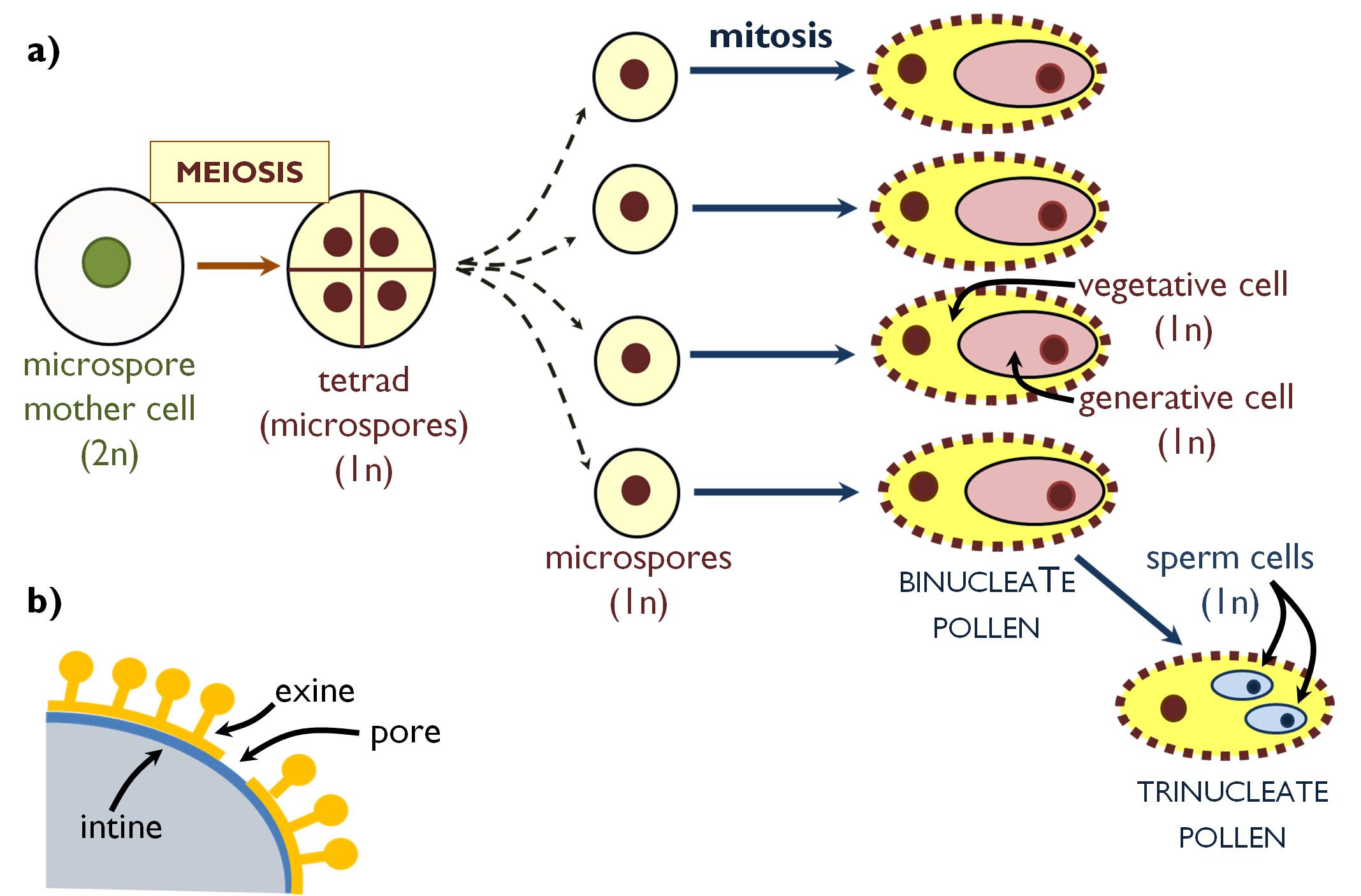
Figure 4. Microsporogenesis and microgametogenesis in angiosperms (a);
the structure of the pollen wall (b).
4.4.3. Megasporogenesis and megagametogenesis in angiosperms
Female spores of angiosperms are born within the evolutionary descendant of the macrosporangium, called ovule. The ovule is attached to the placenta with the funicle (Figure 5). The main part of the ovule is composed of sterile diploid cells, this is the nucellus, which is covered by a two layered capsule, the integuments. At the tip of the ovule, a pore is formed by the integuments, where the pollen tube can penetrate into the ovule. This opening is the micropyle. One of the diploid cells of the nucellus, situated close to the micropyle isolates itself from the surrounding cells by a callose wall. This cell is the megaspore mother cell (megasporocte). Ovule containing the mother cell is called immature. All cells of the immature ovule has a genetic material completely identical with that of the mother plant.
The megaspore mother cell divides by meiosis to produce four megaspores. Further development of the megagametophyte may be different among the angiosperm species, here the most frequent type is discussed (Polygonum-type embryo sac). This type of gametophyte is the product of one single megaspore (monosporic embryo sac), thus the other three spores decay. The megaspore undergoes three mitoses and so gives rise to eight haploid nuclei. Then the cell walls are developed, and seven cells are formed that comprise the embryo sac (megagametophyte). At the micropilar pole is the egg cell apparatus, composing of the haploid egg cell in the centre and two haploid synergids. On the other pole of the embry sac, three haploid antipodal cells are present. The largest cell of the megagametophyte is the central cell the nucleus of which develops via the fusion of two haploid ones. (Thus, the central cell is diploid!)
Once the embryo sac has developed, the ovule is mature and ready for fertilisation. In contrast with the male gametophyte, embry sac remains within the tissues of the mother plant, i.e. closed in the pistil.
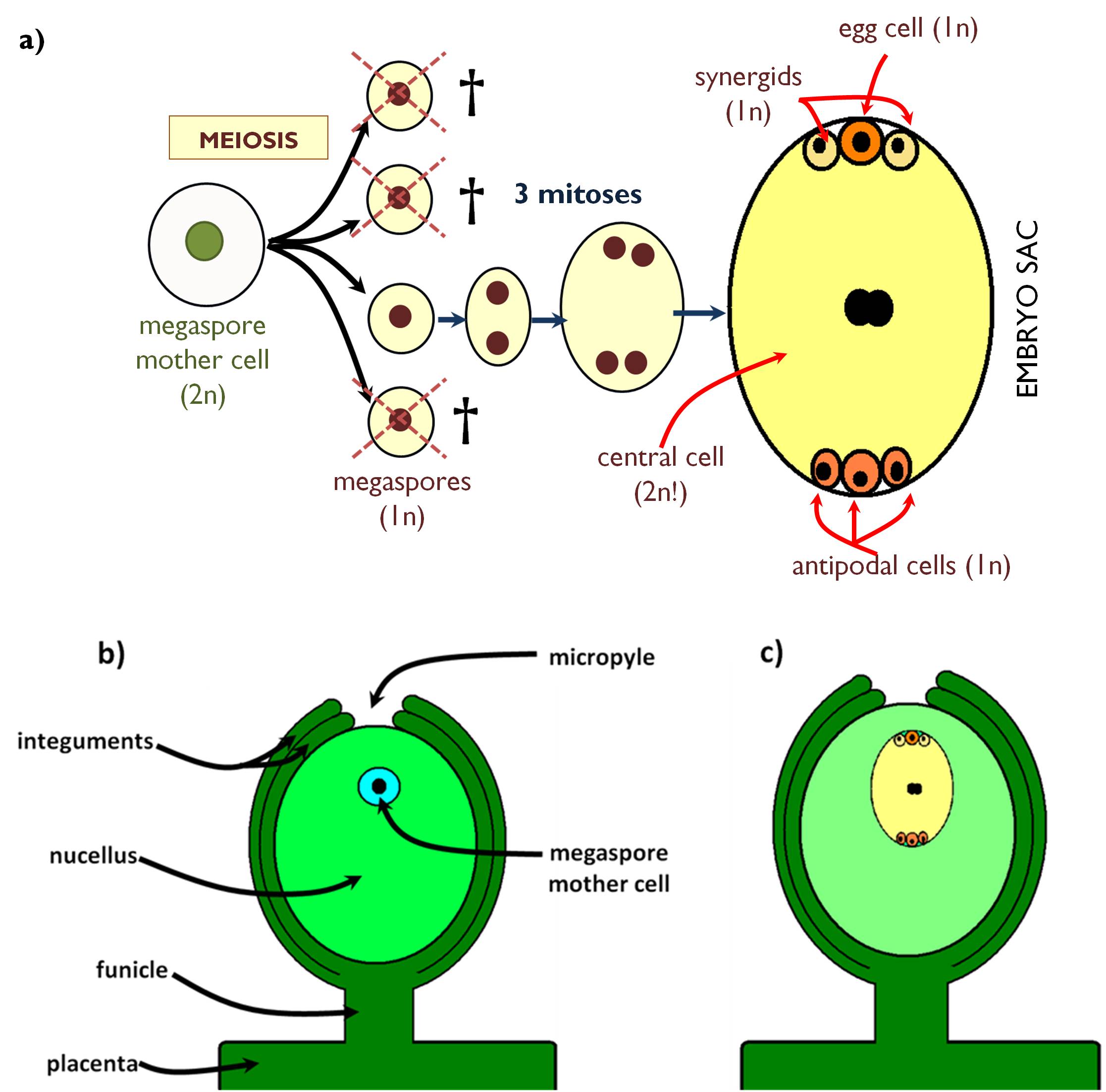
Figure 5. Megasporogenesis and megagametogenesis in angiosperms (a);
the structure of the immature (b) and mature (c) ovule.
4.4.4. Pollination
Pollination means the transfer of pollen grains from the anthers onto the stigma of the pistil. Most flowers – in order to ensure genetic variability – are autosterile, i.e. sperm cells of the pollen grain cannot fertilise the egg cell of the same individual. Autosterility has a genetic background, and occurs on several different levels. (For instance, in some species pollen cannot germinate the pollen tube on the stigma of the same flower, or the tissues of the style ‘reject’ the pollen tube that decays in the pistil.)
Pollen grains only scarcely get directly onto the stigma without any help. More frequently, some outer factors (wind, water, animals) aid the process of pollination. The method of pollen dispersal reflects the ecological environment the plant is adapted to, and it requires morphological and anatomical adaptations from the plant.
Flowers of several autogamous species do not even bloom when pollen germination occurs (chleistogamy). In these flowers (e.g. Viola species), pollen tubes are produced within the anthers, penetrate through the anther wall, and then it enters the pistil (usually through the stigma). Chasmogamy means alternative ways of self pollination, since these flowers are open when pollination occurs. For instance, the developing pistil of the florets of the species in the family Asteraceae grows through the tube of the fused anthers (called synandryum). By this time pollen grains are released from the anthers, thus they adhere to the outer (lower) surface of the stigma. This event does not mean self pollination, since this surface is not susceptible for the pollens. The developed pistil then spreads the lobes of the stigma and waits for the arrival of alien pollens onto the upper side. However, if no xenogamy happens, the lobes become to wither, curl up around each other, and thus own pollen grains attach to the susceptible stigma and self-pollination occurs.
From the anthers of anemogamous plants, polen grains are released into the air and they are taken to the stigma by air currents. Flowers of several anemogamous plants bloom prior to foliation (e.g. Corylus, Acer negundo) so that the leaves do not hinder the pollination. Should flowering take place concurrent to or after foliation, flowers are situated at the end of the shoots, emerging from the foliage (e.g. Populus,Fagus, Carpinus, Betula). Perianth of anemogamous flowers is inconspicuous, vestigial (e.g. Poaceae, Amaranthaceae) or absent (Salix, Populus). Filaments or the pedicels of male flowers are rather long and mobile, anthers produce a high amount of small and light pollens. Pollen grains of pines (Pinaceae) bear air bladders, i.e. small chambers filled with air between two layers of the pollen wall that reduce the density of the pollen and thus enhance their dispersal. The stigma of the anemogamous angiosperms is remarkably extended, plumose or brush-like so that to trap as many pollen as possible.
The most diverse way of pollination is zoogamy, i.e. pollen dispersal by animals. Most frequently pollen grains are carried by insects (enthomogamy), but birds (ornithogamy), snails (malacogamy), bats (chiropterogamy) or other small mammals may act as pollinators. Mostly, enthomogamous flowers provide nutriments for the insects in the form of nectar produced by nectaries situated within or in the close vicinity of the flowers (e.g. Rosaceaea, Lamiaceae) or by producing excess amount of pollen grains rich in valuable proteins (e.g. Papaveraceae, Paeoniaceae). These species attract the pollinators by appearing colours and by an odour released form osmophores. Should the zoogamous flowers be small and inconspicuous, they aggregate to form a dense, flower-like inflorescence (pseudanthium) (like species of the family Asteraceae, or the species Cornus florida and Scabiosa ochroleuca) or these inflorescences are attached to conspicuous bracts (e.g. in the family Araceae or Euphorbiaceae). Baiting colours are not necessary within the spectra sensible for the human eye. Seemingly white flowers may have patterns visible in UV light. Bird pollinated flowers of the tropics have vivid (chiefly reddish or orange) colour, yet they do not have any fragrance.
Flowers are quite rarely pollinated by water (hydrogamy). The perianth of these flowers is reduced and inconspicuous, similarly to the anemogamous flowers. The density of the pollen grains produced by flowers blooming under the water surface is equal to that of the water. These pollens are often filiform and don not have exine. In species blooming above the water surface, male flowers often separates from the pedicel and the whole flower swims to the female flowers (e.g. Vallisneria, Anacharis).
4.4.5. From pollination to double fertilisation
Fallen onto a receptive stigma, the vegetative cell of the pollen grain germinates through an aperture or fissure of the wall if the pollen grain (Figure 6a). During this process, the turgor pressure of the vegetative cell increases due to osmotic water uptake induced by the sugary exudate of the stigma. Actually, the pollen tube (germ tube) is the cytoplasmic appendage of the vegetative cell that grows towards the ovules through the tissues of the stigma and the style. Both the nucleus of the vegetative cell and the whole generative cell enters into the tube and swims towards the ovary. The outermost part (i.e. those in the vicinity of the pollen grain) of the pollen tube growing in the transmitting tissue are continuously separated by callose walls (‘callose plugs’), then these regions degenerate as well. Generative cell of binucleate pollens divides by mitosis within the pollen tube to produce the two sperm cells. (Trinucleate pollen grains already contain the two sperm cells that directly enters the germinating pollen tube.)
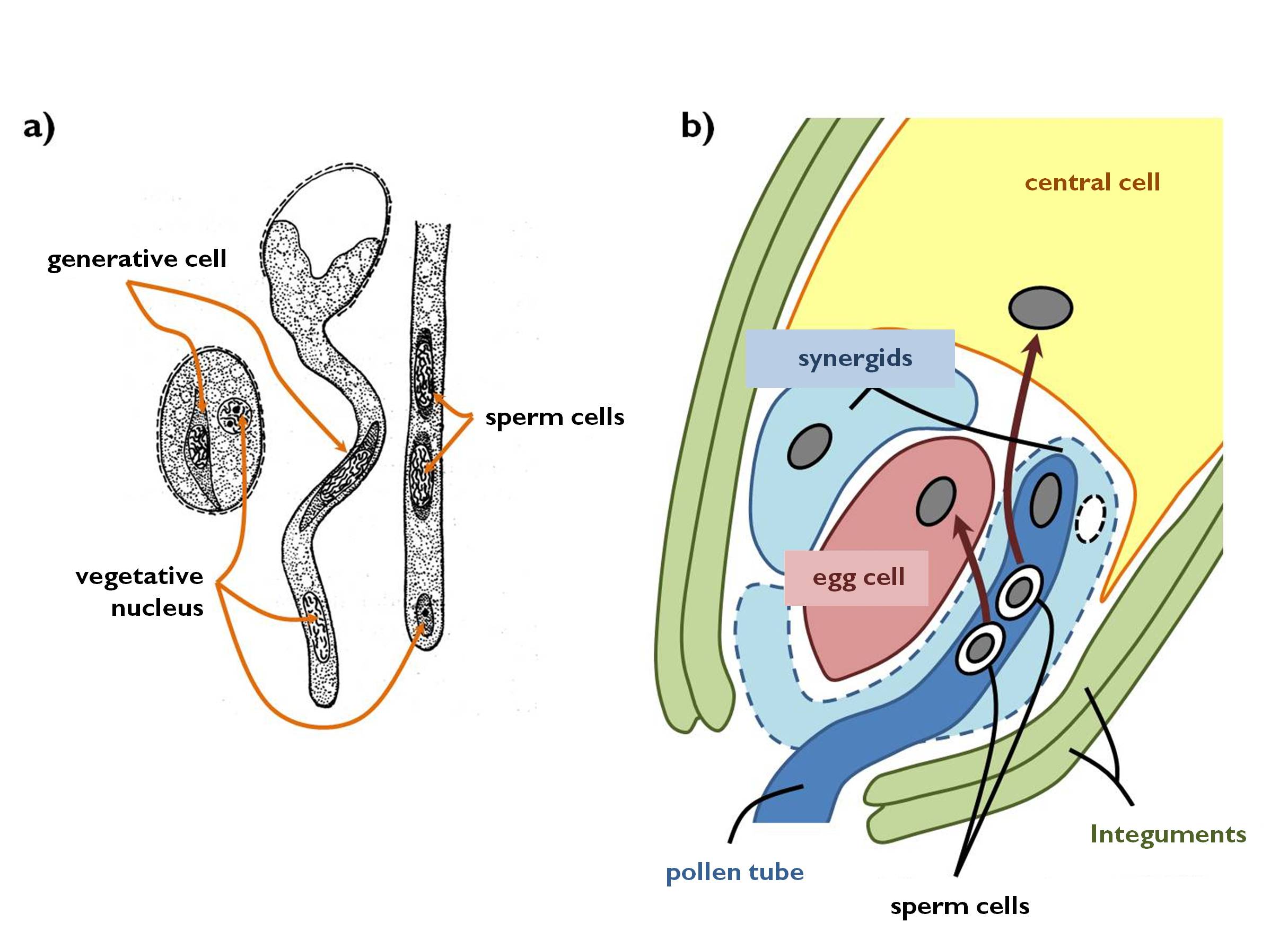
Figure 6. Germination of the pollen tube (a)
and the process of double fertilisation (b).
(a - based on Kárpáti)
In the process of double fertilisation (amphimixis) one of the sperm cells fuse with the haploid egg cell to produce the diploid (2n) zygote, whilst the other sperm cell unites with the diploid central cell. The zygote develops into the embryo (later into the germ). The triploid cell originating from the central cell gives rise to a triploid (3n) nutritive tissue called secondary endosperm. Subsequent to fertilisation, the integuments of the ovule develop into the seed coat, while the nucellus evolves into another kind of nutritive tissue, the perisperm consisting of diploid cells genetically identical with the mother plant. As a result, the ovule is transformed into the seed (Table 4). In the angiosperm flower, from pollination to fertilisation only some hours pass by.
Table 4. Changes resulting from double fertilisation. (Between the dashes ploidy levels are indicated, A stands for structures genetically identical with the maternal sporophyte.)
|
Pistil: |
|
||
|
outer epidermis of the carpel /2n/ |
ovary wall (carpel) |
|
pericarp |
|
mesophyll of the carpel /2n/ |
|
||
|
inner epidermis of the carpel /2n/ |
|
||
|
Ovule: |
|
||
|
integument /2n/ |
|
||
|
nucellus /2n/ |
|
||
|
egg cell /1n/ |
|
||
|
central cell /2n/ |
|
||
4.4.6. Fruit
The fruit is a reproductive organ produced exclusively by angiosperms. Its principal roles are protecting the seeds and later serving their dispersal.
After fertilisation the tissues of the pistil alter: the wall of the ovary develops into that of the fruit (pericarp) (Figure 7). According to the three distinct tissue regions of the carpels, pericarp also consist of three obvious layers: the outer epidermis of the ovary evolves into the exocarp, the mesophyll is transformed into the mesocarp whilst the inner epidermis lining the ovarial chamber produces the endocarp ( Table 4). In several species floral tissues other than the ovarial wall (e.g. the receptacle, the basal parts of the perianth) also fuse around the seeds so that to protect them more safely. These are called false fruits.
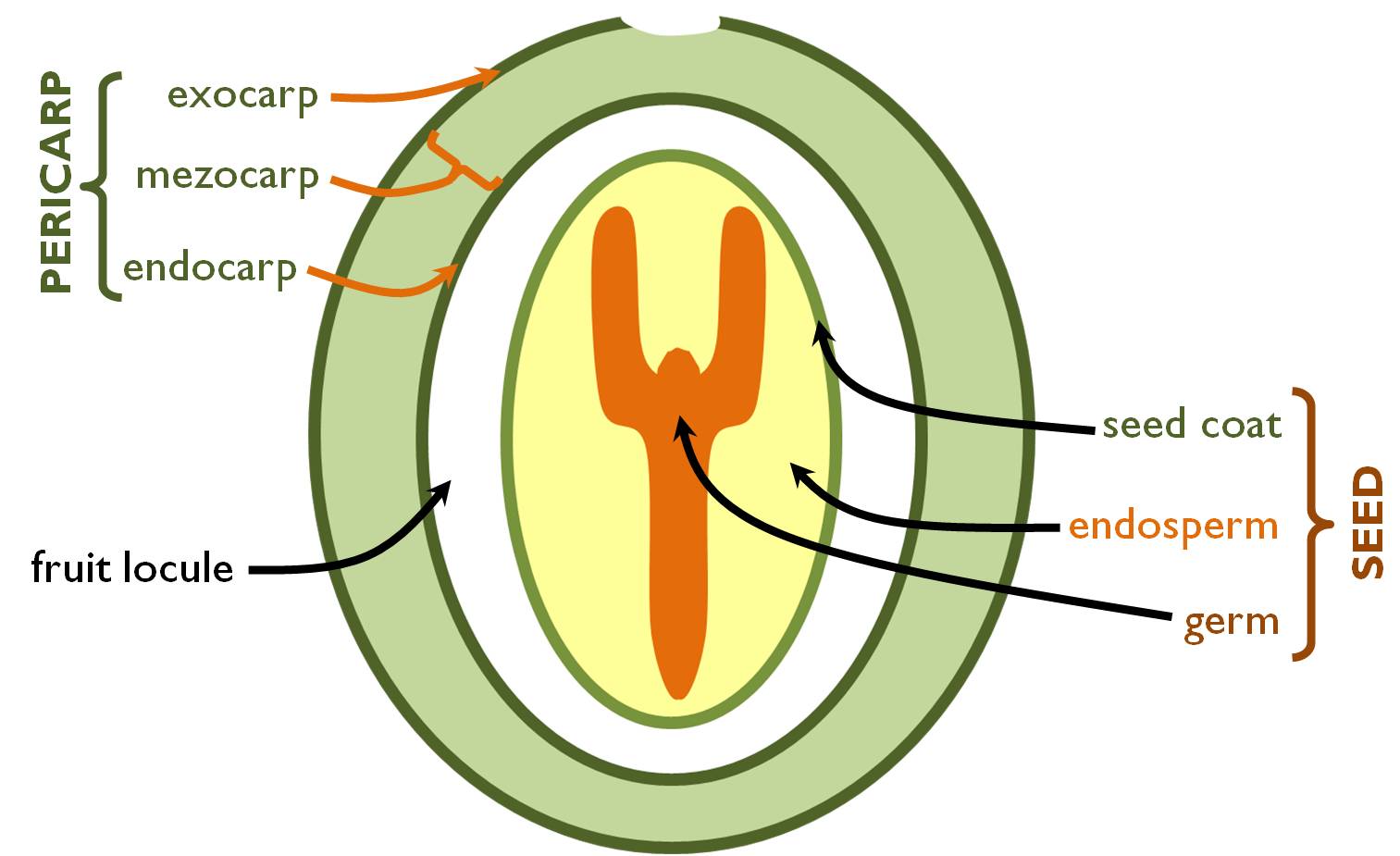
Figure 7. Structure of the fruit and the seed.
(Green coloured parts are diploid tissues genetically identical with the mother!)
Depending on the water content of the fruit containing the mature seeds we distinguish fleshy and dry fruits. If each layer of the fruit wall opens to release the mature seeds, the fruit is dehiscent, in other cases it is called indehiscent. Schizocarpic fruits comprise a special group of indehiscent fruits, because their walls remain closed, yet the fruit breaks up into individual mericarps (e.g. the nucule of the families Lamiaceae and Boraginaceae or the diachene of the family Apiaceae).
4.4.6.1. Histology of the fruit
Due to their various ontogeny, histological structure of fruits (and false fruits, as well) differ considerably. However, there are some general features of the pericarp anatomy in different fruits.
The exocarp of almost all fruits is a unilayered epidermis bearing stomata, trichomes, a quite thick cuticle and a wax layer. An interesting property of this layer is that cells remain mitotically active for an admirable length of time: they continuously divide with anticlinal walls throughout the fruit development.
The mesocarp is the most variable layer of the pericarp. It always contains rudimentary collateral closed vascular bundles (i.e. the nerves of the carpel) that nourish the tissues of fruit wall. In fleshy fruits, mesocarp is composed mostly of storage parenchyma (pulp) containing accumulated storage products, organic acids, sugars, pigments or secondary metabolites. The mesocarp of dry fruits is thinner, and it is often supported by distinct layers of sclerenchyma (e.g. in acorns and nuts). (Obviously, supportive tissues may also be present in the mesocarp of fleshy fruits.) In dehiscent fruits, the mesocarp contains specialised cell layers responsible for breaking up the fruit wall. In these tissues, water loss of the desiccating fruit wall results a stretching force that dehisces the pericarp.
The endocarp in the majority of fruits is a modified, single-layer inner epidermis. However, the anatomy of this region often modifies, because several general epidermis functions are unnecessary in this position. Cells of the endocarp may turn parenchymatic and thus partially comprise the pulp (e.g. in berries). The endocarp of the stone fruit is composed of stone cells (brachisclereids) forming a ‘stone’ that protects the seed within. The endocarp of some fruits (e.g. caryopsis, diachene) fuses with the seed coat.
The locule of the fruit may be filled with air (e.g. Capsicum annuum) or liquid (e.g. Solanum lycopersicon), yet a loose tissue deriving from the endocarp may also be present here (e.g. Citrullus lanatus), as well as the fleshy epidermal hairs of the endocarp (e.g. Citrus sinensis).
4.4.7. Seed4
The seed is the evolutionary innovation of the gymnosperms (i.e. of the seed ferns). It serves the protection, nutrition and dispersal of the embryo within. The latter function is principally important in the gymnosperms and angiosperms with dehiscent fruits. The seed develops on the mother plant (maternal sporophyte), from the ovule as a result of the (double) fertilisation. When separated from the plant it enters a dormant period ( seed dormancy) as a response to the hormone abscisic acid. The dormancy is terminated by the beginning of germination.
4.4.7.1. Anatomy of the seed
Seeds are always enveloped in the seed coat, a coverage deriving from the integuments of the ovule. This is usually bilayered consisting of an outer testa and the inner tegmen. The seed coat bears a distinct marking, the hylum that establish as a result of separation from the funicle, that may be of characteristic size and/or shape (e.g. in the family Fabaceae, or Aesculus hippocastanum, Cardiospermum halicacabum). Around the hylum, further typical structures may be present. Should the funicle grow onto the seed coat (e.g. in ase of anatropous ovules), after the separation a marked ridge (raphe) is visible. Seeds dispersed by ants bear a swollen structure here accumulating nutrients (oils, proteins) for the insects. These are called elaiosomes.
The seed coat may bear appendages of various shapes serving mainly the seed dispersal. Such structures are the trichomes (e.g. Salix, Gossypium) or the wings (e.g. Pinus). Outside the seed coat, some seeds possess also a vivid, fleshy aril, which is the derivative of the funicle (and not that of the integument). Aril is present on the seeds of Taxus baccata, Euonymus or Paeonia, as well on those of the species of the family Nymphaeaceae. The red, fibrous aril of the nutmeg (Myristica fragrans) is sold as a spice called ‘mace’.
The outermost layer of the seed coat is a unilayered (or often modified) epidermis. Beside epidermal structures (cuticle, trichomes), cells accumulating mucilage may also occur in it (e.g. Linum, Sinapis). Within the inner, parenchymatci layers supporting elements (sclereids, fibers) may be born, especially in seeds released from dehiscent fruits. Sometimes, remarkably reduced vascular bundles are also observed here. Rarely, fleshy parenchyma may also be present in the seed coat, like in the sarcotesta of the seed of Ginkgo biloba. (The inner, sclerenchymatic layers of the seed coat of the ginkgo comprise the tough sclerotesta.)
The interior of the seed contains the embryo (germ) and the endosperm. Storage parenchyma feeding the embryo may be of various origins 5. The principal storage tissue of gymnospermous seeds is the haploid primary endosperm (1n) formed by the cells of the female gametophyte. In angiosperm seeds, this function is served by the secondary endosperm (3n) originating from the central cell of the embryo sac or the direct derivatives of the maternal tissues (nucellus) of the ovule (perisperm – 2n). Nevertheless, not all angiosperm seeds contain any of the mentioned tissues. In these seeds, the cotyledon of the embryo (i.e. diploid embryonic cells) accumulates the storage compounds. The proportion of different types of storage tissues in the seed may be different in certain angiosperm species, some of them containing more than one kind of storage tissue in its seeds. However, some taxonomic groups have their own type of nutrient accumulation:
-
seeds with primary endosperm : gymnosperms;
-
seeds with secondary endosperm : Poaceae, Liliaceae, Solanaceae, Apiaceae, Polyagonaceae;
-
seeds with perisperm : several genera of the family Caryophyllaceae;
-
seeds with both secondary endosperm and perisperm : Piperaceae, Cannabidaceae, Amaranthaceae, Zingiberaceae;
-
seeds with storage tissue in the cotyledons : Fabaceae, Fagaceae, Rosaceae, Asteraceae, Cucurbitaceae.
4.4.8. Dispersal of fruits and seeds
The most important role of fruits and seeds are reproduction and dispersal. So that to reach this goal, it is important for the offspring (the germ) to get as far from the mother plant as possible. The dispersal of seeds and fruits remarkably determine the dispersal strategies and so the area of the species. As a result of evolution, several different ways of propagule dispersal developed. These are mostly achieved by the morphological or anatomical modification of the seed (in case of dehiscent fruits) or the fruit(wall) (in case of indehiscent fruits).
Anemochory means dispersal by wind. Quite low is the number of such flowery plants that possess so minute and light seeds which do not require any special structures for their dispersal (e.g. species of the family Orchidaceae or the genera Petunia, Begonia and Orobanche). More frequently, the wind rolls all the plant (e.g. Eryngium, Salsola) or a broken part of the plant (e.g. Papaver), and seeds are scattered during this movement. Larger fruits and seeds possess modified structures for their dispersal. These can be ‘wings’ of the fruit wall (e.g. Fraxinus, Ulmus, Acer) or the seed coat (e.g. pines, Campsis, Catalpa), yet such role may also be played by bracts (Tilia, Carpinus). Anemochory may also be served by special hairs. Such trichomes may be born on the seed (e.g. Gossypium , Asclepias, Salix, Populus) or they can be attached to the fruit. The achenes of Clematis and Pulsatilla species are dispersed by the hairy style, the hairy glume aids the dispersal of the caryopsis of Stipa, whilst hair-like reduced sepala ( pappus) floats the cypselas of the daisy family (Asteraceae). The stone fruits of Cotinus coggygria are dispersed by the hairy pedicels of infertile flowers of the panicle.
Flower-dispersed (hydrochore) fruits have water-resistant pericarp and an inner cavity filled with either air or a fluid of density lower than that of the surrounding water (e.g. Nuphar sp., Nymphaea sp., Trapa natans). Floating fruits are flown away by the currents, sometimes to an admirable distance of several hundred kilometres (e.g. Cocos nucifera, Lodoicea seychellarum).
Animals may also take part in propagule dispersal (zoochory) in various ways. In case of endozoochory, fleshy (or rarely dry) fruits are consumed by the animal, yet seeds pass unchanged through the digestive tract and they germinate following the defecation. Fruits of these species are of vivid colour, high nutrient content, scented and often of large size (e.g. Vitis, Rubus, Malus). Seeds of some endozoochore plants are incapable of germination unless the digestive enzymes loosen their seed coat in the digestive tract of the dispersing animal. More frequently, the fruit or the seed clings to the fur or the plumage of the animal (epizoochory) with clinging hairs, hooks (e.g. Arctium, Xanthium, Hibiscus, Galium aparine) or the gluey mucilage covering the epidermis (e.g. Viscum, Linum). A considerable proportion of seeds and fruits is born away and gathered by the animals (synzoochory). Meanwhile, some of the propagules are shed and thus the species is dispersed. Such way of dispersal is often served by ants (myrmechochory). The seed of a myrmechochore plant bears a small protein- and/or oil-rich appendage called elaiosome or caruncula that provides nutrients for the animals (e.g. Viola, Chelidonium, Corydalis, Ricinus).
A further group consists of the self-dispersal fruits, when the fruit forcibly ejects the seeds by means of various morphological modifications of the fruit wall. Usually the dehiscing fruit wall provides the force for the seed ejection (e.g. in case of legumes or the capsules of Impatiens, Viola, Geranium or Oxalis species). The fruit of the squirting cucumber (Ecballium) suddenly detaches from its pedicel when ripe, and a stream of mucilaginous liquid of high pressure squirts out of the fruit cavity together with the seeds.
4.5. Plant embryogenesis
A common feature of terrestrial plants (Embryophytes) is that their zygote does not develop directly into the plant, but primarily it gives rise to the embryo consisting mostly of meristematic cells. The process of embryogenesis in certain taxonomic groups may be quite different, thus here we basically discuss the way how the majority of embryos in the eudicots (Magnoliophyta, Rosopsida) develop.
By the divisions of the zygote the total plant can be constructed. Its derivatives may give rise to any tissue of the plant. Such mother cells are called totipotent. The descendants of the totipotent cells become more and more differentiated, i.e. their mitoses can produce only the tissues certain organs. These are the pluripotent cells. The cells produced in the organ primordial are still meristematic, but their derivatives can develop only into certain tissue systems of the organ – these are called multipotent cells. Interestingly enough, the differentiation level of the plant cell can be changed by hormonal effects: multipotent cells can be transformed into pluripotent or even totipotent ones (for example in case of injuries or prior to the initiation of somatic embryos in a vegetative organ).
Embryogenesis begins with the unequal division of the zygote within the embryo sac (Figure 8). This results a smaller apical cell of dense cytoplasm and a larger, highly vacuolated basal cell. Due to this event the proembryo becomes polarised: the apical cell gives rise to the majority of the embryo, whilst the basal cell develops into the suspensor. The basal cell of the two-celled proembryo divides unequally, again with a transversal wall (perpendicular to its longitudinal axis). The lower, large cell is the suspensor cell that later divides several times transversally to produce the suspensor, which anchors the embryo to the mycropylar pole of the ovule and transports the nutrients (and regulatory compounds) from the mother plant to the embryo. The upper daughter cell (adjacent to the apical cell) is the hypophyseal cell that divides into four with longitudinally oriented walls. Later these derivatives take part in the formation of the root pole (radicle) of the embryo.
The apical cell of the two-celled proembryo divides twice with longitudinal walls and gives rise to the quadrant (four-celled) stage. The synchronous transversal division of the four cells results the octant (eight-celled) stage. The cells of the octant divide periclinally to enlarge the proembryo, which from now can be divided into two distinct regions, into the outer (superficial) layer and the central mass of meristematic cells. In this period the embryo is spheric in shape (globular stage). Superficial cells divide anticlinally to form the protoderma (that later gives rise to the epidermis), while the cells of the inner region divide both anticlinally and periclinally. The surface of the central region is composed of highly vacuolated cells that later produce the ground meristems, while the inner, less vacuolated cells continuously become arranged axially at give rise the procambium.
In the next period, two meristematic cells groups within the globular proembryo intense mitotic divisions begin to produce the primordial of the cotyledons. Later, these primordia are visible as small protrusions on the surface of the proembryo, what makes it heart-shaped. With the ongoing development of cotyledons and the elongation of the embryonal axis (actually that of the hypocotyl) the proembryo enters the torpedo stage. Meanwhile, mitotic divisions become restricted to the two terminal poles of the embryo: to the embryonal shoot tip (plumule), between the cotyledons and the embryonal root tip (radicle) adjacent to the suspensor6 7. (The radicle is the derivative of the hypophysis.) The stem of the embryo is called hypocotyl below the cotyledons (right till the root), while between the cotyledons and the first primordial leaves it is called epicotyl.
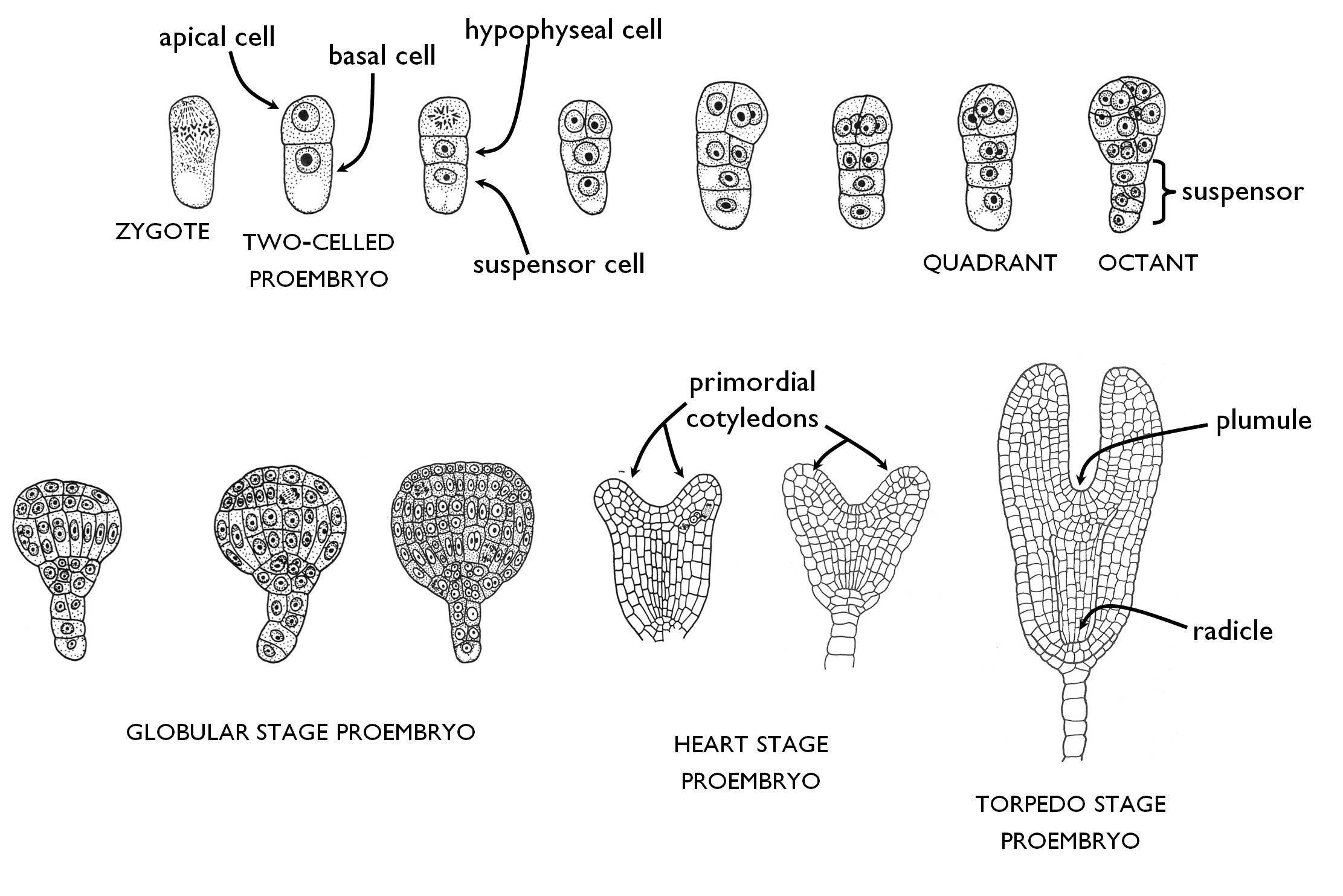
Figure 8. The general way of embryogenesis in eudicots (Rosopsida).
(Based on Gyurján)
References:
Erős-Honti Zs (2012): A kertészeti növények alaktana. Egyetemi jegyzet. Budapesti Corvinus Egyetem Kertészettudományi Kar, Bp.
Gyurján I (1996): Növényszervezettan. Egyetemi jegyzet. ELTE Eötvös K., Bp.
Haraszty Á (1998): Növényszervezettan és növényélettan. 6. kiad., Nemzeti Tankvk., Bp.
Jacob F, Jäger EI, Ohmann E (1985): Botanikai kompendium. Natura, Bp.
Kárpáti Z, Görgényi L, Terpó A (1968): Kertészeti növénytan I. Növényszervezettan. Mezőgazdasági Kiadó, Bp.
Mauseth JD (2008): Plant Anatomy. The Blackburn Press, New Jersey.
Mishra SR (2009): Understanding Plant Reproduction. Discovery Publishing House Pvt. Ltd., New Delhi.
Sárkány S, Haraszty Á (1995): Növényszervezettan biológia-kémia, biológia ill. pszichológia-biológia szakos hallgatók részére. Egységes jegyzet. Nemzeti Tankvk., Bp.
Sárkány S, Szalai I (1966): Növénytani praktikum I. Növényszervezettani gyakorlatok. 3. kiad., Tankvk., Bp.
1 A special way of layering is applied for herbaceous plants. In this case the bottom of the densely grown plant is graded with the rooting medium, what facilitates the development of adventitious roots in the bottommost nodules of the stems.
2 Interestingly enough, both long- and short-day plants sense the length of the dark period (the ‘night-length’), despite their naming. This is proven by the fact that short-day plants cannot flower if the durable dark period following the short day is interrupted by short light flashes. Nevertheless, among such conditions, long-day plants flower, irrespective of the length of the light period.
3 This description refers to the more common type called wet stigma. Besides, some species have dry stigma covered by cuticle (and a thin layer of proteins). In these species the adhesion of pollen grains to the surface of the stigma is aided by the sticky outer layer of the pollen grain.
4 Here we stress that the term ‘seed grain’ applied in agriculture does not always refer to seeds in the botanical aspect. Seed grains may be the stones of stone fruits (e.g. peach, apricot), the mericarp of the species of the carrot family (e.g. fennel), or any indehiscent fruit containing a single seed, like the achene (aster family – sunflower) or the caryopsis of grasses (e.g. maize, wheat). Similarly, the fused capsules of beets (Beta ) are also sold as seed grains.
5 Storage material is mostly starch accumulated in amyloplasts, but it may be oil stored in oleasomes or protein present as aleurone grains in the cells.
6 Some authors use the terms ’root pole’ and ’shoot pole’ for the embryo, and they apply the terms ’radicle’ and ’plumule’ only after the primordia of the subsequent root and shoot are initiated.
7 Fern embryos contain no radicle but only the shoot pole. They are unipolar embryos – instead of being bipolar embryos like those of the flowering plants (discussed in the text).

