Pests and diseases in button mushroom cultivation
Author: András Geösel
A number of diseases caused by viruses, bacteria and different microscopic fungi can occur in mushroom cultivation. The larvae of pests (such as Phoridae and Sciaridae species) cause direct damage by feeding on the mycelia and the fruitbodies, but by carrying and spreading the pathogens and other pests, they have an indirect role as well.
Hygiene in mushroom growing requires special attention and careful execution for more reasons. In one hand, cultivated mushrooms are often consumed raw or unprocessed, which increases the risk of foodborne contaminations. In the other hand, the growing cycle of mushrooms is very short (the whole cultivation cycle is as short as 4-5 weeks, while a button mushroom fruitbody gets ready to be picked in 4-6 days). Since the pathogens are becoming resistant to the chemical products, the emphasis has to be put on prevention rather than treatment.
Viral diseases
Viral diseases of cultivated mushrooms (mycoviruses) can cause huge yield losses. The two most important viruses are the La France Isometric Virus (LIV) or La France disease and the Mushroom Virus (MVX). It is assumed that viruses have long time been present in the Hungarian mushroom cultivation as well, only their detection and identification was impossible without modern technology. In Hungary, the first double strained RNA molecules (which indicate the presence of the MVX virus) were found in 2000 by immunoblotting and RT-PCR analysis. The identification of viruses was earlier difficult, since it can only be done in laboratories using modern PCR methods. From the two most common viruses, one causes significant damage in button mushroom cultivation. Other virus diseases occur in oyster and shiitake cultivation, but they have not yet been detected in Hungary.
La France Isometric Virus (LIV)
Symptoms
The disease causes yield loss, but usually no other symptoms can be seen. The shape and size of the matured fruitbodies are the same (only their amount is much less), but the pinheads develop differently (their stem elongates and the cap is undersized, which symptoms are similar to that caused by high CO 2 level). The caps open and discharge the spores earlier than usual. A dark brown discoloration appears in the middle of the fruitbody and the stem often gets soggy.
Prevention
Since hybrid strains are used in cultivation, the virus appears less often. After the symptoms are detected no treatment is effective, thus prevention must be in focus. The first thing to do is to keep the growing area, the surfaces, tools and equipment and the clothes of the workers clean (or even sterile). Fruitbodies showing any symptoms should be removed from the growing area to prevent spore dispersal, since spores are virus vectors. In order to minimize the spore count in the air, the ventilation systems of the growing houses should be equipped with HEPA filters. The floors have to be carefully cleaned and sterilized if possible. Before removing the spent compost from the growing house, the material should be cooked out to prevent cross-infestation of other growing areas. Nowadays the La France disease occurs quite rarely.
Mushroom Virus X (MVX)
Symptoms
The virus was first detected in button mushroom cultivation in England, in the mid 1990’s and it spread rapidly throughout Europe. The first symptom is that the colonization of the compost by mycelium is not complete, the mycelial growth in the virus infected areas is altogether slower and the forming pinheads are irregular. Other symptoms of the MVX:
- longer and protracted flushes,
- earlier spore spread,
- glassy or brownish spots on the fruitbody,
- distortion of the stem and the cap,
- significant yield loss and less first class mushroom.
Prevention and treatment
Since no chemical product is effective against viruses, prevention is highly important. Spores are vectors of viruses, thus their spreading should be minimized (by removing the over matured fruitbodies and equipping the ventilation system with HEPA filters). Other vectors are mushroom flies, which transport not only viruses but also spores and mycelia of pathogens. The virus was detected in Hungary as well. By burning the infested compost and quickly sterilizing the growing area, the spreading of the virus was successfully blocked (so far). In case the above mentioned symptoms occur, immediate laboratory analysis is necessary to prove the presence of the virus.
Preventive actions against viruses
In the growing area:
- Casing soil should be stored in regularly cleaned, sanitized rooms.
- Hygiene during casing is not only important because of viruses, but for the prevention of other diseases.
- Post crop cooking out of the growing room is effective against viruses as well.
- Filtering the air of the growing area helps minimizing spore spread.
- Keeping the picking equipment, machines and clothes of the pickers clean and sterile is important.
During compost preparation:
- The spawning machine should be sterilized before using. Overpressure in the spawning area helps keeping spores (and pests) away from the compost.
- High efficiency filters have an important role not only in the growing house but in the compost plant as well.
- The surfaces and the walls of the rooms, as well as all equipment and machinery have to be properly sanitized.
Bacterial diseases
Amongst many bacterial diseases (e.g. Pseudomonas gingeri – ginger blotch disease and Pseudomonas agarici – drippy grill) of cultivated mushrooms, Pseudomonas tolaasii (bacterial blotch) is the most common.
Bacterial blotch disease
Pathogen: Pseudomonas tolaasii
Symptoms
This pathogen can cause symptoms on both the white and cream type button mushrooms, in each developmental stage (from pinning to picking). On the surface of the caps 1-2 mm, shiny, yellowish-brown or brownish-black spots with distinct margins appear. Later on the spots form blotches and spread on the cap surface all the way down to the stem. Unlike in case of dry bubble disease, the blotches never sink into the tissue.

Symptoms of bacterial blotch disease on button mushroom
Biology of the pathogen
The bacterium lives in the casing soil (since it occurs in peat moss in nature), in the limestone and in the beet potash too. It can also be found in the waste resulted by picking and also on the necrotized pinheads. The bacterium is spread by spores, mushroom flies, mites, by the hands and tools of the pickers and even by the water used for irrigation. The ambient conditions (especially the high humidity) of a growing house are ideal for bacteria.
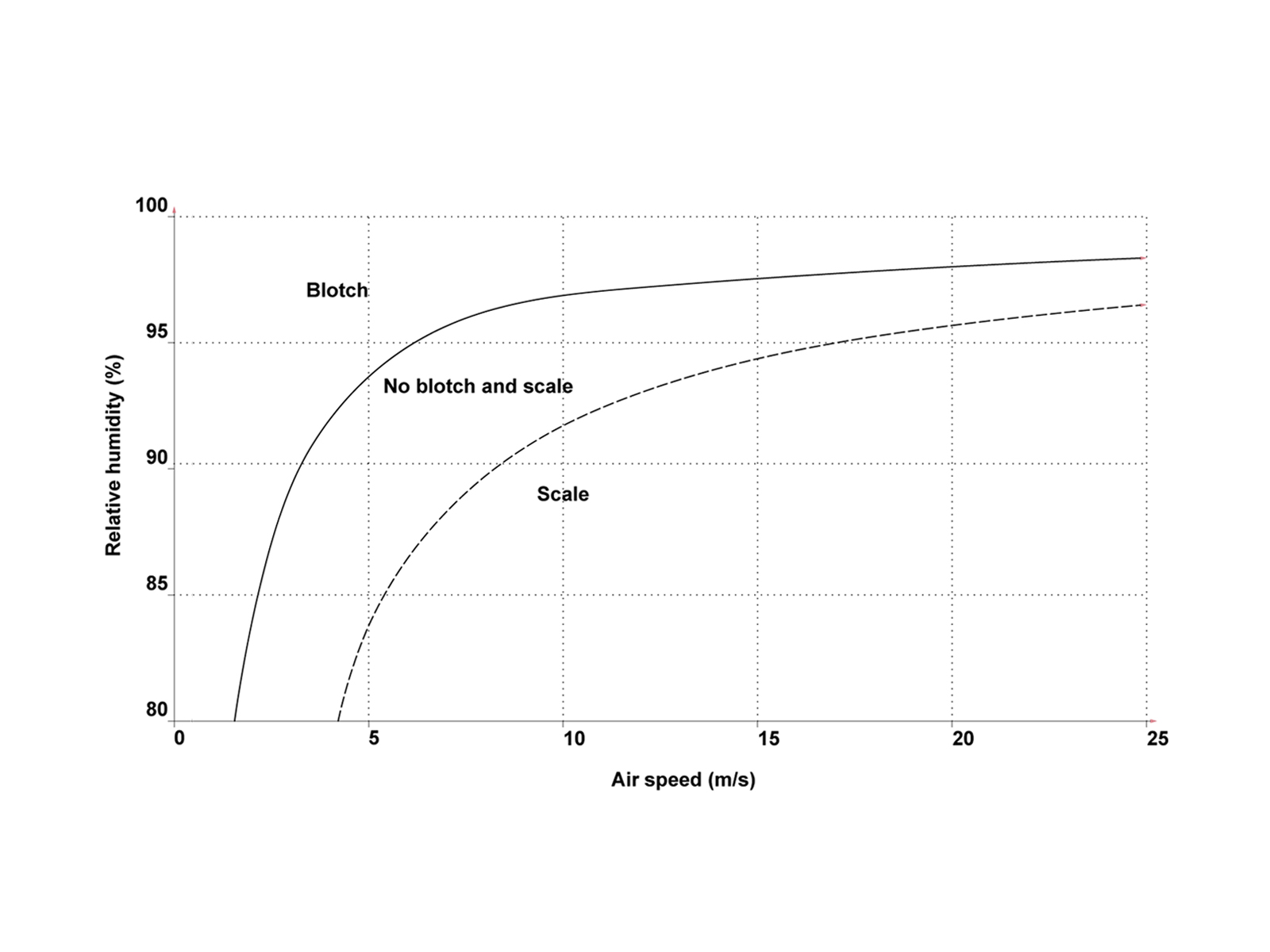
Occurrence of blotch disease and scaling on relation of air-flow and humidity
The number of bacteria in the casing soil grows rapidly. When the bacteria count reaches 106 in 1 g of casing (which happens quickly on 20˚C or above), the symptoms of the infection can be detected within a few hours. The spreading of the bacterium is especially fast is the surface of the pinheads or matured fruitbodies are moisture or even wet. The toxin produced by the pathogen dissolves the mushroom cells, which is why, as a defense mechanism, the mycelia start to produce enzymes that cause the brownish coloration of the spots and blotches. If a bacterium infested fruitbody left together with the healthy ones after picking, the disease could spread even in the +2˚C cooler, lowering the quality of the product.
Sources and spreading of the disease
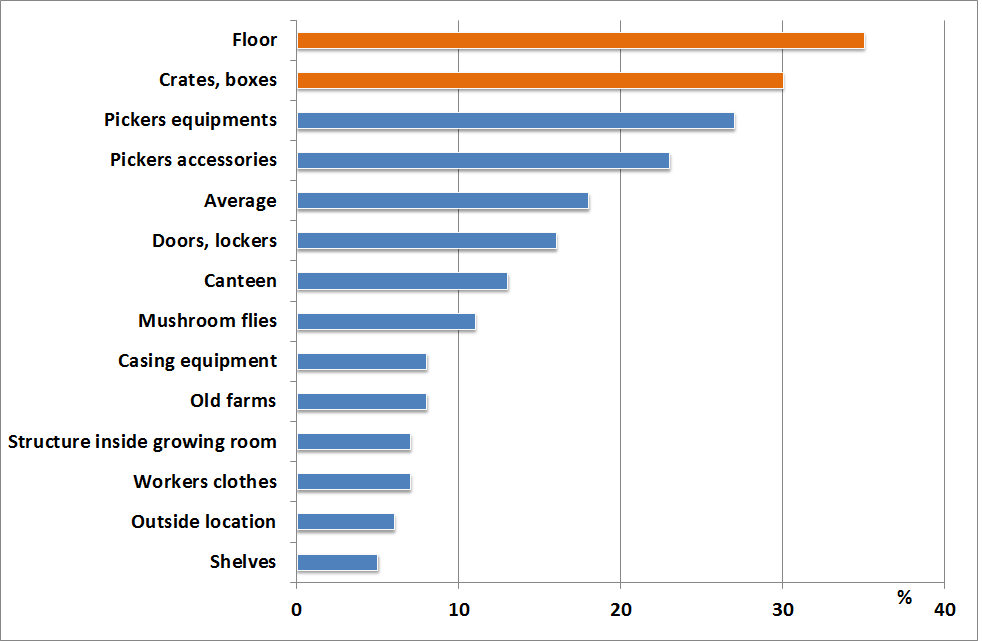
The sources of the bacterium are the infested casing soil and the dust, which gets into and settles on the surfaces of the growing area. In some cases, although the bacteria can be found in the casing soil, they do not cause any symptoms. So far it has not been explained which conditions induce the disease, but it is proven, that too high temperature together with high (above 85%) relative humidity during cropping increase the risk of infestation. Moreover, if the surface of the fruitbodies cannot dry within 2-3 hours after irrigation, the spreading of the disease throughout the growing room is going to be very quick.
Prevention and treatment
Pseudomonas tolaasii is usually present in each growing room. Although prevention is not really an option, the possible damage can be minimized by some sanitary actions.
- The prime source of the disease is the casing soil. That is why the mixing of its ingredients (peat moss, limestone, beet potash etc.) should be done in a clean, closed area under hygienic conditions.
- In growing houses where the disease frequently occurs, the growers steam (cook out) the casing soil, which in one hand has high costs, and additionally destroys the useful micro organisms (e.g. Pseudomonas putida) that are later on needed for spawn run and fruitbody formation. This case P. putida has to be added to the mixture before casing.
- Sudden temperature changes have to be avoided, as it causes drops forming on the surface of the fruitbodies. That is why the mushrooms should be dried by proper air-flow after irrigation too.
- Evaporation of the mushrooms depends on compost and air temperatures, on relative humidity and air-flow. Inadequate air-flow together with high humidity lowers the intensity of evaporation and is in favor of the spreading of the bacteria. On the other hand, in the air-flow is too intense and the humidity is lower than optimal, scales appear on the fruitbodies.
- No bactericides can be used in mushroom cultivation and there aren’t any Pseudomonas-resistant cultivars available for button mushroom growing.
It has to be well-known, that bacterial symptoms are always an indicator of poorly-managed cultivation technology.
Pathogenic fungi
There are two types of pathogenic fungi, which cause yield loss in button mushroom cultivation: competitor and parasitic species. Competitor fungi compete with the mycelium of the cultivated mushroom for nutrients and water in the compost and in the casing soil. These species are also called indicator moulds, as their occurrence indicates problems with the substrate (low quality ingredients, inadequate heat treatment, low quality casing soil etc.). If these species appear in the compost or casing soil during spawn run, no effective treatment is available, thus the emphasis has to be put on prevention. Due to recent innovations, the development of new techniques in compost preparation and applying more effective hygienic actions in the growing houses, the diseases caused by pathogenic fungi in mushroom growing are less likely to occur.
Parasite moulds are more common than competitors. They also compete for the nutrients, but at the same time cause so much damage in the mycelia of the cultivated mushroom that many times the colonization cannot be completed.
A number of Trichoderma species can be isolated from the compost, casing and the fruitbody, such as T. koningii, T. viride and T. harzianum. A common characteristic of these species is that they all produce a high number of spores in different shades of green. The species causing the most significant problems and yield loss in mushroom cultivation is the Trichoderma aggressivum.
Green mould
Pathogen: Trichoderma aggressivum f. europaeum Samuels et W. Gams
Trichoderma aggressivum f. aggressivum Samuels et. W. Gams
Green mould causes the most damage in phase III. composts. Since the majority (around 90%) of mushroom growers use phase III. compost, these species are one of the most significant pathogens in mushroom cultivation. T. aggressivum produces toxins, which damage the mycelium of the cultivated mushrooms. The optimal temperature is 22-26˚C for the pathogen (the different biotypes prefer even higher temperatures). The mould is white at the beginning, but as soon as the spores start to discharge (in 2-4 days), it turns green.
Trichoderma can get into the compost in the plant, during spawning or bulk heat treatment. If infestation happens during the spawning of the compost, the yield loss is likely to be 100%. As the mycelia of the pathogen is white (just like the mycelia of the cultivated mushroom), spore spread is the first stage when the disease can be detected (because of the green spore color). This usually happens after the compost left the plant and got to the grower.
In many cases, the pathogen starts feeding on the spawn freshly mixed to the compost, because it is the most nutrient rich ingredient, creating the false impression that the spawn was the source of the disease. During phase III. compost is transported to the grower, the temperature can easily get above 28˚C, which is in favor of Trichoderma mycelial growth. Supplementation (added during spawning) also increases the risk of infestation, as the nutrient rich substrate is optimal for the pathogen.
Symptoms
Continuous, usually round patches appear first in the compost, than on the surface of the casing soil. The patches turn green within a few days. Mites, such as Pygmephorus mesembrinae and P. priscus feed on the spores of Trichoderma species. If these mites are present in the growing room, the compost is infested by Trichoderma for sure, even if no green patches are visible.
Trichoderma can also cause symptoms on the fruitbody: a number of light-brown or grey spots of 5 mm size, with no distinct margins appear on the stem and the cap. The symptoms of the fruitbody can be mistaken to that of the cobweb mould disease. The pathogen survives on the moisture, unsterilized surfaces (e.g. tools and wooden equipment) and is easily spread amongst growers by wrappings, boxes etc. The source of infection can also be residues left on the surface of the compost after picking.


Symptoms of Trichoderma aggressivum on the casing soil
Prevention and treatment
- Prevention starts at the compost plant. The only effective action is to prevent the spores of the pathogen getting into the compost and surviving the heat treatment. Trichoderma spores cannot survive the heat peak (57-58˚C), but only if the ammonia level is at least 450 ppm. The mushroom compost is most likely to get infested during the cooling period (that follows the heat treatment), at spawning and in the first week of spawn run. That is why spawning has to be most sanitary procedure of compost preparation; the conveyor belt, the tools and machinery have to be thoroughly sterilized before spawning and the air has to be filtered. Formaldehyde can be used for sterilization.
- Transportation of the compost (bulk compost or bags and sacs, especially phase III. compost) has to be done by special care, so the temperature does not get too low or too high. Also the vehicles have to be cleaned and sanitized previous to loading.
- At the end of a crop in Dutch-type houses, the whole room (including the shelves filled with spent substrate and casing soil) has to be cooked out with 65-70˚C steam for at least 9-12 hours. This way the spores will not survive in the gaps of the shelves and on the surfaces. In cellar cultivation and in mushroom tents cooking out is not an option. In these types of facilities chemicals (formaldehyde, Virocid) have to be used.
- Another important thing to do in the growing area is to prevent vectors (especially mites) entering, for example by applying filters on the ventilation system.
- The clothes of the pickers have to be changed, washed or steamed for 30 minutes daily. All the equipment used for picking (knives, boxes, buckets, trays, stands etc.) have to be sterilized each day.
Besides green mould, the other three most important diseases of the button mushroom caused by microscopic fungi are the dry bubble disease, the wet bubble disease and the cobweb mould. These pathogens occur in every mushroom producing country; only the scale of the damage caused by them is different.
Dry bubble disease
Pathogen: Lecanicillium fungicola (Preuss) Zare & W. Gams
Although the pathogen was renamed as part of the Lecanicillium genus in 2008, it is still referred to as Verticillium fungicola (Preuss), which is the previous and widely known name. The pathogen appears quite regularly and causes serious yield losses in every type of button mushroom cultivation. Many strains ofLecanicillium fungicola are known, in Europe the Lecanicillium fungicola var. fungicola (previously known as Verticillium fungicola var. fungicola) is the most common.
Symptoms
Unlike the Mycogone perniciosa (wet bubble disease), Lecanicillium fungicola var. fungicola causes various symptoms on the button mushroom. These symptoms can occur any time during the cropping cycle depending on the spore count, season, type of growing house, quality of the casing soil and cultivated strain etc.


Symptoms of dry bubble disease on button mushroom (spots and “bubbles”)
If the infestation occurs in the early stage of fruitbody development, 20 mm sized undifferentiated masses (“bubbles”) form. In case of wet bubble disease, this size could be 100 mm or even bigger. Fruitbodies showing symptoms and healthy ones can grow even next to each other. If the fruitbody gets infected in a later stage of development, the deformation is usually uneven: a part of the cap or the stem or both suffers distortion. A thin, whitish-grayish layer of mycelium covers the surface of the infested fruitbodies, under which the mushroom tissue gets discolored and has a dry touch.
The disease could also cause spots on the surface of the caps. The 10-20 mm spots are initially light grayish-blue, then turn brown and the middle of the spot soaks, creating a crater-like form. The spots then get covered by smooth grayish mould, which is the sign of spore spread. The caps shrink, the spots dry and get cracked. The spots caused by Lecanicillium often get mistaken to those of caused by bacterial diseases, but while the spots of dry bubble disease dry eventually, the bacterial ones remain shiny because of the ooze produced by the bacteria. The infected fruitbodies develop in 8-10 days (either in groups or alone), they are always dry and do not have any unpleasant smell.
Biology of the pathogen
Lecanicillium species have sticky conidiospores. Unlike the spores of Mycogone, Lecanicillium conidia stick together and also covered by a layer of ooze, which aids sticking to dust, pests, tools and other vectors. The spore-group often start to develop wherever they stick (in the cracks of the floor or the gaps of the shelves), from where they are transported by water during irrigation or by the tools and equipment. Some pests feed on the mycelium and spores of Lecanicillium but some of the spores do not get digested and start developing from the excrement. Other pests (e.g. Sciaridae and Phoridae mushroom flies) carry the spores on the bodies. The spores can also get into the growing area by infested vehicles, boxes clothes or even by visitors. Neither the Lecanicillium, nor the Mycogone can survive in the compost or grow through the casing soil, thus the cultivated mushroom gets infected when the spores of the pathogens get close or next to their developing mycelia. Lecanicillium spores are mainly spread by irrigation and picking.
If the dry bubble disease is detected in the first flush, it is likely that the third flush is going to be completely infected.
Spreading of the dry bubble disease on an Irish mushroom farm (Piasecka et al., 2011)
Wet bubble disease
Pathogen: Mycogone perniciosa (Magnus) Delacr.
Besides the dry bubble disease, wet bubble disease is one of the most important diseases of button mushrooms that cause significant yield losses. Another species, Mycogone rosea infests wild grown button mushrooms.
Symptoms
The principal symptoms are the “bubbles”, which are deformed, undifferentiated fruitbodies. The bubbles may appear in the first flush (or even 1-2 days earlier) and could reach the size of 100-140 mm. The surface of the bubble is smooth and white at first, but turns brown within a few days. Besides the mould, drops form on the surface as well, which are small and yellowish at the beginning, then grow and turn brown. The drops are produced by bacteria living on the mould. The bubble is soft and has a very unpleasant smell.


Symptoms of wet bubble disease on button mushroom
The bubbles appear 10-14 days after the mycelium of the cultivated mushroom gets infected. The initial symptoms are small, white spots of thick, fluffy mycelia, which eventually turn brown. In rare cases, wart-like bumps can be observed on the surface of the caps.
Biology of the pathogen
Mycogone perniciosa has two types of spores: conidia (one cell, short lifespan and thin cellular wall) and chlamydospore (thick cellular wall, long lifespan – 3-8 years!). Although conidia are light and can be transported by the wind, both forms are more likely to spread by water (irrigation). The pathogen lives in the soil, thus the primary source of infection is the casing soil. The casing material often gets infected before getting into the growing house, this case the symptoms appear as early as during the first flush. The pathogen cannot be found in the compost but can survive in the growing area (in the cracks and gaps or on the equipment and tools). Once the pathogen infests the culture, it is spread by irrigation easily. Although the spores are not sticky (unlike in case of Lecanicillium), Mycogone spores can be spread by the clothes or the tools of pickers all around the farm.
Prevention of the dry and wet bubble diseases
- Boxes used for picking can be sources of infections if they are taken into the growing room without sterilizing.
- The primary source is the casing soil. The room where the casing material is kept and handled has to be thoroughly cleaned and sanitized. The tools used in the room should not be used elsewhere. A foot dip (for shoe sterilizing) has to be placed in the door (just like in every room where cropping takes place), and everyone have to step into it before entering the rooms.
- The casing material can be heat treated but never sterilized. The optimal heat treatment lasts 30 minutes on 60˚C, during which the number of pathogens lower but the useful microorganisms survive. The chemical disinfection is also could have a good results.
- The fruitbodies showing symptoms have to be picked first thing the day and removed from the growing area in closed plastic bags. Common salt is poured on the bubbles and then removed while wearing gloves or using paper towel. Irrigation should only be started after all infected fruitbodies are removed from the room.
- For prevention (but not for treatment) Sporgon 50 WP (3 g/m2) can be used at casing, at the first irrigation following casing and at the last irrigation before ruffling in 1.5 g/m2 dose.
Cobweb disease
Pathogen: Cladobotryum dendroides (Bull) W. Gams et Hooz., syn. Dactylium dendroides, telemorph syn. Hypomyces rosellus (Alb. & Schwein.) Tul. & C. Tul.
Cobweb disease is common in button mushroom cultivation. Prevention is getting less effective since the pathogen has developed resistance against the active agent benzimidazole and getting resistant to Sporgon 50 WP as well. The disease spreads fast and causes severe yield loss. It infests each developmental stage, but most cases appears during the middle or end on the cropping cycle. The most damage is caused when the infection happens during or before casing and the symptoms appear in the first flush.
Symptoms
The name of the disease derives from the main symptom, which is quite distinctive: the cobweb-like mycelium covers the fruitbodies and the surface of the casing soil. 1-2 days later the fine mycelium becomes thicker, more veil-like and turns pink. The cobweb appears on the surface of the casing only if it is already colonized by the mycelia of the cultivated mushroom. High amounts of spores appear on the round, thick mycelium patches. The infected, mycelia covered fruitbodies later shrink and turn brown. The cap deforms, the gills thicken and in 10-12 hours the fruitbody leans down and starts to decay. The smell is unpleasant and strong.
Dark or light brown colored spots may appear on the surface of the caps. These spots look similar to those caused by Trichoderma. The spots grow together with the cap, until it covers the whole cap and part of the gills. The mushrooms start to rotten under the mycelium cover.


Symptoms of the cobweb disease on the fruitbodies and the surface of the casing soil
Biology of the pathogen
The optimal conditions for the pathogen are 25˚C, 90-95% relative humidity and pH 5-6. The asexual spores of the fungi are the 2 (or more) celled conidia. The conidia are transported by the air-flow, water and vectors, such as mushroom flies or even by the pickers. Unlike the spores of Lecanicillium, Dactylium spores are not sticky. Smaller pieces of mycelia could be transported just like the spores.
The pathogen lives in the soil and infests wild grown mushroom species as well. The primary source of the disease is the soil surrounding the mushroom farms, the casing soil, the air (which contains pieces of mycelia or spores) and the pickers themselves. The disease appears usually at the second or third flush. Unlike Lecanicillium and Mycogone, this pathogen can grow into the casing soil and even into the compost, thus the substrate can also be the source of infection. Irrigation and high humidity together with insufficient air-flow aids the rapid spreading of the disease, since the surface of the caps cannot dry in time.
Prevention and treatment
The infected fruitbodies and the mycelia patches are covered with moisture paper towels and then salt is poured over them. This way the spores cannot discharge and spread the disease. Sporgon 50 WP can be used as a preventive action.


Cobweb and wet bubble disease infected fruitbodies covered with paper towels and salt
Pests
The pests causing the most problems in mushroom cultivation belong to the Diptera order, to the families Sciaridae, Phoridae and Cecidomyidae. The primary damage is done by the larvae, which feed on the mycelia, while the secondary damage is due to the fact that adults spread eggs of other pests, mycelia and spores of pathogenic fungi and microorganisms, such as bacteria and viruses on their bodies.
Mushroom flies
Lycoriella solani Winnertz, L. inguenua /Dufour/, L. auripila /Winnertz/, Bradysia brunnipes Meig
The most significant pests of button mushroom cultivation. In nature they can be found feeding on wild grown mushrooms, living in composts, manure and decaying wood, from where the adults find their way into the mushroom farms.
The larvae
The larvae feed on the mycelia in the compost and the casing. The infested parts of the compost and the casing soil are moisture, smell bad and only weakly colonized by the mycelium. The females lay eggs in the not yet spawned or colonized compost. The first larvae appear in the first flush and feed on the base of the fruitbodies, where they eat away the mycelia, which causes the mushrooms to stop growing, turn brown and get slimy. The mature larvae bore through the stem (and sometimes the cap) of the bigger fruitbodies. The tunnels created by the pest are filled with the faecal matter of the feeding larvae, which cannot be seen from the outside (only when the mushrooms are cut in half).

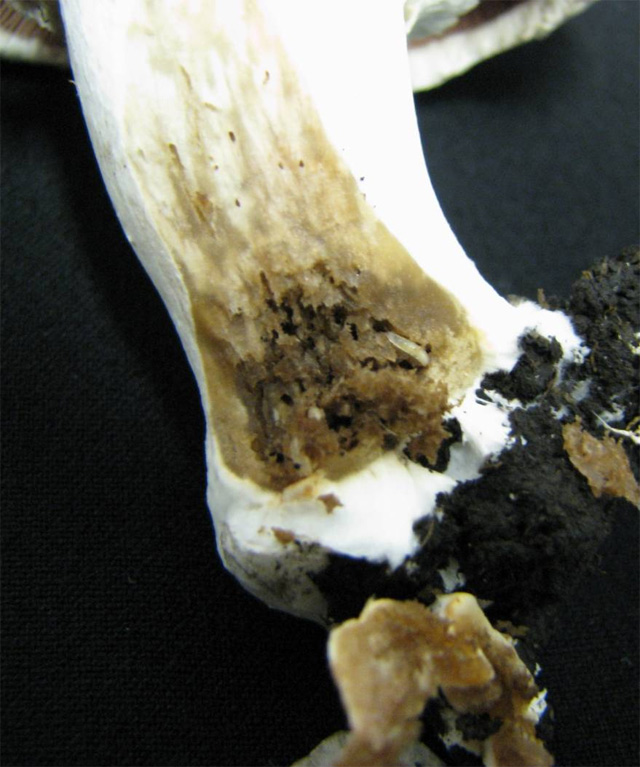
Fruitbodies damaged by larvae of Sciarid flies
The adult
By spreading a number of diseases (and sometimes even other pests), adult mushroom flies cause secondary damage in mushroom cultivation. Unlike Phorid species, Sciarid flies clean themselves rarely. The mites get on them (as many as 30 mites at the same time), and get transported by the flies. Additionally, bacteria can be found on the mites, thus not only the pests, but the microorganisms are carried by the flies as well. Sciarid flies have an important role in spreading the spores of the dry bubble disease (Lecanicillium fungicola).
The anatomy and biology of the pests
The mature larvae are 8-12 mm long maggots, which have distinctive chewing mouthparts on their shiny black heads. 2 generations can develop, if the females lay eggs around spawning.

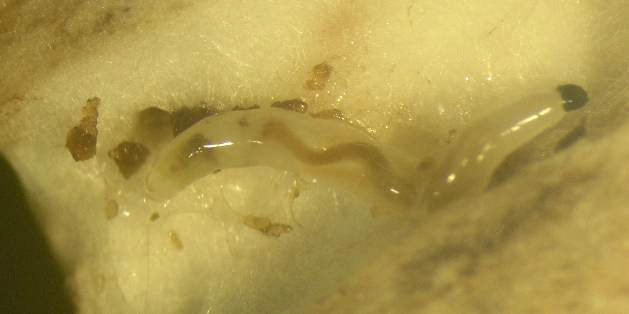
Sciarid larvae with the characteristic shiny, black head
The adults are 3-4 mm black flies with long, thin antennae which are held characteristically (in 45˚).

Sciarid fly with the quite distinguishable antennae
The female lay 140-170 eggs in the not yet colonized compost or in the casing soil (during cropping, even on the surface of the fruitbodies). The eggs can either be found one by one or in groups (10-15 pieces). On 24˚C the pest reaches adult state in 18-22 days. The temperature is above 24˚C during colonization, thus the larvae could appear as soon as the first flush begins. During cropping the development from egg to adult takes more time (35-38 days), as the temperature is lower, only 18˚C. The more larvae and adults are present, the more crop loss can be expected. Sciarids, in contrast to Phorid species which are seasonal pests, usually stay on the farm throughout the whole year.
Prevention and treatment
Chemicals or biological products are available against mushroom flies. Adequate production method and strict sanitary actions are essential in prevention.
- Once the compost is colonized, the females do not lay eggs there (since the growing mycelia inhibit larval development), thus the most important thing is to prevent flies getting near the phase II. compost. That is why the rooms where colonization takes place are overpressure. In less modern mushroom houses the doors, windows and ventilation ducts have to be covered with screens.
- Grower using phase II. compost should cover the bags or sacs with paper or plastic sheets after placing them on the floor or on the shelves. This way the female flies cannot lay eggs into the compost (only on the covering material). The covers should only be used once.
- Any type of waste has to be removed from the surroundings of the growing area. The spent substrate cannot be left on the farm.
- Dimilin 25 WP (active ingredient: diflubenzuron) can be used against larvae as prevention. It can be added to the water following the first irrigation in 4 g/m2 dose.
- The biological product (Nemacel, Nemasys-M) used against mushroom flies contains the eelworm species Steinernema feltiae and is also effective.
- Sticky traps can catch a large number of flies. Electric traps are not as effective, since the flies avoid light.
Phorid (hump-backed) flies (Megaselia halterata, M. nigra)
Phorid flies are common all over the world. They feed on decaying and composting organic materials, wastes. Since Phorid flies reproduce quickly, they can cause serious problems short after appearing in the crop. The flies can fly long distances (10 km in 24 hours). That is why disposing of the spent compost has to be done by special care. Phorids are seasonal pests, as they only appear from spring until autumn. Although the descriptions of Megaselia halterata and M. nigra are similar, there are differences between the feeding of the two species. M. halterata larvae feed solely on mycelium in the compost or in the casing soil, but do not bore into the fruitbodies, whereas M. nigra larvae tunnel the mushroom tissue right after emerging.
Megaselia halterata
The larvae are rarely seen, since feed in the compost or in the casing. The adults can be found easily especially in summer and early-autumn. These flies are one of the most effective vectors of the dry bubble disease (Lecanicillium fungicola), as they can carry hundreds of spores on their bodies (in spite of the fact that they clean themselves more often than Sciarid flies). Nowadays the indirect damage caused by the disease spreading adults is more significant than of that of the larvae.
Phorid flies are small (2-3 mm) hump-backed pests with practically inconspicuous antennae. The larvae are cream white, 1-6 mm long legless maggots with a pointy head and a blunt rear end. Unlike in case of Sciarids, Phorid flies do not have black heads. The female lays around 50 eggs near the mycelium. After that they live (and spread diseases) for weeks. Phorids do not fly below 13˚C, which explains why they are not infesting the farm from the surroundings during winter.
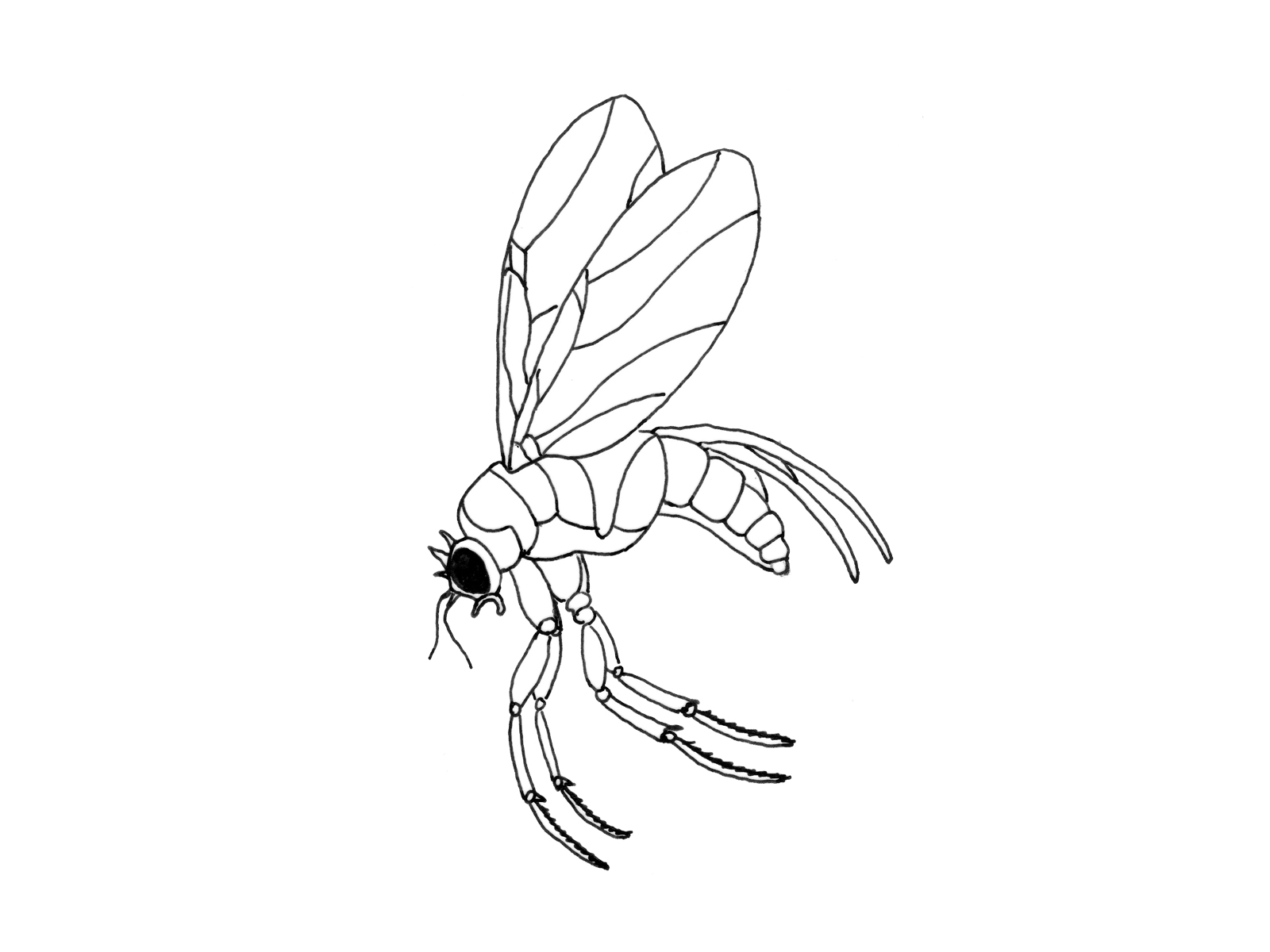
Phorid fly with the characteristic hump back
Megaselia nigra
M. nigra feed on both wild grown and cultivated mushrooms. The female lays eggs on the casing next to the developing fruitbodies or directly on the mushrooms, so food is instantly available for the emerging larvae. As a result of larvae activity, the fruitbodies get completely tunneled (both stem and cap) and finally the collapse. After a serious infection nothing is left on the surface but a mass of mushroom tissue, faecal matter and a group of larvae. Sciarids never bore into the cap, while Phorids tunnel through the entire fruitbody. M. nigra is slightly bigger and darker than M. halterata, otherwise they look the same. One female is capable of laying 500 eggs, 20 at a time and 40 within 12 hours. Larvae emerge in 3 days (on 18˚C), feed for 5 days before turning into pupa. On 24˚C the insect develop from egg to adult in 13-14 days and lives for 36 days. Since females prefer laying eggs on surfaces that get natural light, the occurrence of the pest is more likely in tents (and not in cellars or artificially lid Dutch-type houses).
Prevention and treatment
Just like in case of all pests (and diseases) prevention is first. The females can smell mycelia, start to look for it and try to get into the growing area. This can be prevented by thoroughly covering the openings with screens.
- Every used material (plastic or paper covers, bags and especially the spent compost) have to removed and transported far from the farm, since they contain the pest (in different developmental stages), which can infest new crops.
- Electric traps should be used, since the flies prefer light. Yellow sticky traps are also effective in catching flies.
- Since the larvae hid in the mushrooms and do not come to the surface, the only opportunity to use chemicals against them is to add Dimilin 25 WP (4 g/m 2) to the first irrigation as prevention.
- The biological products containing eelworms are not effective against Phorids.
Cecid flies (Heteropeza pygmaea, Mycophila speyeri)
Heteropeza pygmaea and Mycophila speyeri are the most common Cecid flies. The larvae of H. pygmaea are white and they occurred more frequently in button mushroom cultures in Hungary, while the other species have orange colored larvae and can be found mainly in oyster cultivation.
The unusual reproduction method of Cecid’s is called paedogenesis, where the larvae do not turn pupa (then adult), but they become ‘mother-larvae’ and give birth to 12-20 daughter larvae. The mother larva reaches 3-4 mm in 6 days and by the end of its development, contains 1 mm sized embryos. The reproduction rate is so high that one handful of casing soil could contain 18 000 larvae in different developmental stage. Near the end of the cropping cycle (around the 3rd wave) the mycelium in the compost and in the casing starts to age. This time the maggots turn into adult-larvae, then pupa and finally adults emerge. In this period the flies can be caught by electric traps, which attract thousands and thousands of them.
The sources of infection are usually the peat and organic wastes. The larvae suck the mycelial cell content, damage the developing mycelium, which slows colonization and cause the pinheads to die. The larvae usually are only detected by the time they caused significant mycelium damage. H. pygmaea larvae appear after irrigation. The swarm out of the beds/bags, congregate on the surface of the mushrooms and the casing soil, or on the shelves and bags and move like “waves” in writhing mass. The larvae feed on the middle part of the fruitbody (where the stem and the cap meets) and on the gills. Many bacteria can be found on their bodies, which then (as secondary damage) cause brown, discolored stripes to form on the stem and the gills.
The tiny (approximately 1 mm) yellowish adults can rarely be seen because of the specific reproduction of the pest. Depending on the developmental stage, the daughter larvae are around 1-3 mm long. The young larvae are always moving. If the amount of food is insufficient for the large number of larvae, they move to the surface of the casing and stick together in a big (30 mm) mass. This is the stage when they become clearly noticeable and they can easily be spread throughout the whole growing area.
Prevention and treatment
- The sticky larvae are easily spread by clothes and equipment, thus these materials have to be washed, sterilized or changed regularly. Workers should proceed with the daily work from the newest crop towards the oldest, so that the infections are not spread.
- Peak heat during compost preparation and cooking-out by the end of crop are effective against Cecids.
- Spent substrate has to be removed from the farm.
- Casing materials should be stored and handled in clean, closed rooms.
Eelworms (Nematoda)
The source of nematode infections are usually the organic materials used for compost preparation and mushroom growing, such as horse and chicken manure, wheat straw and casing soil, but an infection can also derive from unhygienic conditions (waste and dust in the growing area or in the compost plant). Eelworm populations grow quickly, this way they can cause serious damage in a short period of time.
Eelworms are 0.8-1 mm long, transparent worms which prefer moisture conditions. There are two types of nematodes: saprophytes and parasites. Both cause problems in mushroom production. Thorough heat treatment (on 55˚C for 6 hours) during compost preparation prevents infestation from the compost, but the casing material still can be the source of eelworms. Due to advanced hygienic actions nowadays nematodes are not as significant pests as they used to be a few decades ago, that is why no detailed description is given. The following table includes the most important species and the damage caused by them.
The most important eelworm genuses in mushroom production
|
Saprophyte |
Parasite |
|
Caenorhabditis elegans (Rhabditida) |
Ditylenchus myceliophagus (Tylenchida) |
|
Acrobeloides apiculatus (Rhabditida) |
Pseudhalenchus spp. (Tylenchida) |
|
Cephalobus spp. (Rhabditida) |
Aphelenchoides agarici (Aphelenchida) |
|
Diplogaster spp. (Rhabditida) |
Aphelenchoides composticola (Aphelenchida) |
|
Rhabditis spp. (Rhabditida) |
Aphelenchoides myceliophagus (Aphelenchida) |
|
|
Aphelenchoides neocomposticola (Aphelenchida) |


Symptoms of nematode infection in the compost and the eelworm itself under microscope
Mites (Acari)
There are three types of mites in mushroom production: parasites, saprophytes and predators. Parasites feed on organic material, which in case of mushroom production is the mycelium. Usually the feeding of the pest does not cause the host to die, only when the infection is serious. As a result of the feeding of predator mites, the preys die. Saprophytes need decayed (and other) organic materials.
These tiny, spider-like pests are present in the compost and in the casing too. Phase I. compost could contain a very high number of mites, which is one of the reasons why a thorough heat treatment with an efficient peak-heat is necessary for prevention. Since they are virtually invisible to the naked eye, their presence can be left undetected for long. Just like nematode infections, nowadays mite infections are not as common as they used to be. The only exceptions are the red pepper mite species (Pygmephorus spp.). Red pepper mites are not primary pests, but since they feed on spores of Trichoderma species, their presence indicates mould infection (even before the typical green colonies become visible).

Pygmephorus mites on green mould infected button mushroom culture
Yellow garden slug (Lymax flavus)
Slugs prefer moisture environment. It is most likely to appear in cellar cultivation. (That explains why it is a more significant pest in Hungary than in Europe: cellar cultivation is more typical here than anywhere else). The slugs feed on each part of the fruitbody. It not only bites out pieces of the mushroom, but at the same time leaves its slime on the surface. There are chemicals available against slugs (e. g. Delicia). Next to the walls and on the floor, common salt can be poured. The pests are attracted by beer, yeast or wet clothes. By placing traps filled with these materials, growers can keep slugs away from the mushrooms.
Hygiene in mushroom production
Currently there are only two synthetic active agents allowed here in mushroom production (diflubenzuron and prochloraz-Mn). There is another product, which contains nematodes and is used efficiently against Sciarid flies, but is quite expensive. Growers will not be able to use the above mentioned chemicals for long, since in many cases resistance or tolerance evolved. Using non-authorized plant protection products in mushroom cultures is forbidden. If any residue can be detected in the mushroom, the producer can get financial penalty.
In modern (Dutch-type) mushroom houses these chemicals are not necessary, if the hygiene requirements are followed properly. In Hungary, only a smaller part of mushrooms are produced in Dutch-type houses, while most mushrooms are grown in cellars or tents.
Good hygienic practice starts at the beginning: the establishment of the mushroom farm. The farm has to be far from organic waste deposits and landfills, and from forests that can serve as constant source of pests. The farms have to be equipped with changing rooms and social facilities such as washrooms, thus the personal hygiene of the workers can be kept on a proper level. The workers have to be equipped with protective clothing. The strict rules of using chemicals (e.g. formaldehyde) have to be followed carefully.
It is proven that most diseases are spread by the workers throughout the farms. Hygiene has to be taken into account during the organization of work: e.g. picking should be started in rooms with cultures that are in the earliest stage of development and then proceed to the older cultures.
Mats have to be placed at each entrance. The size of the mat should not allow the workers to go around or overstep them. The mats have to be soaked with a contact plant protection product.
Spent compost has to be removed from the farm. It should not let to get into contact with the new compost. Following the removal of the substrate and any remaining organic material (e.g. casing, waste), the room has to be cleaned and all surfaces have to be treated with contact products.
The above mentioned steps are only parts of the hygienic actions required for economical mushroom production. It is worth to spend on preventive actions (cleaning, disinfecting) as hygienic conditions enable more effective, high quality mushroom production.
Check your knowledge!
- Describe the symptoms of dry bubble disease!
- What are the preventive actions of bubble diseases?
- Describe the symptoms of mushroom flies!
- What are the lifestyles of mites?
- What are the preventive actions of Trichoderma?
- Describe the cobweb disease!
- Give at least five examples of improving hygiene!
References
Castle, A.J., Sivanesan, D., Dobbin, C., Rinker, D.L. (2004): Causal Agents of Bacterial Blotch on Cultivated Agaricus bisporus in Canada. Science and Cultivation of Edible and Medicinal Fungi. Proceedings of the XVIth International Congress on the Science and Cultivation of Edible and Medicinal Fungi, (Miami, USA, 14-17 March, 2004), 441-447.
Catlin, N.J., Wuest, P.J., Beyer, D.M. (2004): Green Mold Harbored by Wood: Post-crop Steaming and Preservatives. Science and Cultivation of Edible and Medicinal Fungi. Proceedings of the XVIth International Congress on the Science and Cultivation of Edible and Medicinal Fungi, (Miami, USA, 14-17 March, 2004), 449-458.
Chang, S.T., Miles, P.G. (2003): Mushrooms. The Chinese University Press, Hong Kong, 159-188.
Clift, A., Shamshad, A., Terras, M.A. (2004): Flies and Dry Bubble on Cultivated Mushrooms. Science and Cultivation of Edible and Medicinal Fungi. Proceedings of the XVIth International Congress on the Science and Cultivation of Edible and Medicinal Fungi, (Miami, USA, 14-17 March, 2004), 459-478.
Ehlers, R.U., Hokkanen, H.M.T. (1996): Insect Biocontrol with Non-endemic Entomopathogenic Nematodes (Steinernema and Heterorhabditis spp.): Conclusions and Recommendations of a Combined OECD and COST Workshop on Scientific and Regulatory Policy Issues . Biocontrol Science and Technology, 6, 3: 295-302.
Fletcher, J.T., Gaze, R.H. (2008): Mushroom Pest and Disease Control – A Color Handbook. Manson Publishing, London.
Fletcher, J.T. (2002): Cobweb disease, a new challenge. Mushroom News, 50, 4: 20-23.
Foster, G. D., Amey, R., Bailey, A., Mills, P.R. (2004): Investigation of the Interaction Between Verticillium fungicola and Agaricus bisporus. Science and Cultivation of Edible and Medicinal Fungi. Proceedings of the XVIth International Congress on the Science and Cultivation of Edible and Medicinal Fungi, (Miami, USA, 14-17 March, 2004), 479-487.
Gaze, R.H., Calvo-Bado, L., Challen, M.P., Aide, B., Romaine, C.P. (2000): A new virus disease of Agaricus bisporus? Science and Cultivation of Edible Fungi, 150, 2: 701-705.
Grewal, P.S., Weber, T., Betterley, D.A. (1998): Compatibility of the insect-parasitic nematode, Steinername feltiae, with chemicals used in mushroom production. Mushroom News, 46,4: 6-10.
Grogan, H.M., Tomprefa, N., Mulcachy, J., Holcroft, S., Gaze, R.H. (2004): Transmission of Mushroom Virus X disease in crops. Mushroom Science, 16: 489-498.
Győrfi, J., Fürst, K. (2009): Biológiai védekezés a Sciaridae-legyek lárvái ellen a csiperkegomba termesztésben. Növényvédelem, 45, 1: 29-36.
Győrfi, J., Geösel, A., Póczik, E. (2008): Trichoderma fajok elleni biológiai védekezés a csiperketermesztésben. Kertgazdaság, 40, 1: 7-13.
Győrfi, J., Geösel, A. (2008): Biological Control against Trichoderma Species in Agaricus Cultivation. Proceedings of the 6th International Conference on Mushroom Biology and Mushroom Products, 158-164.
Győrfi, J., Geösel, A. (2009): Trichoderma-fajok a csiperketermesztésben: lehetséges a biológiai védekezés? Növényvédelem, 45, 9: 517-521.
Győrfi, J. (2003): Csiperketermesztés nemcsak vállalkozóknak. Szaktudás Kiadó Ház, Budapest, 5-199.
Győrfi, J. (2011): Kórokozók és kártevők a csiperketermesztésben. (in: Győrfi, J. (szerk): Gombabiológia, gombatermesztés. Mezőgazda Kiadó, Budapest, 191-225.
Halász, K., Geösel, A., Szarvas, J., Bray, M., Hajdú, Cs., Lukács, N. (2007): A termesztett csiperke (Agaricus bisporus LGE.) MVX vírusának kimutatása immunoblott módszerrel. XVII. Keszthelyi Növényvédelmi Fórum kiadványa, Keszthely, 12-16.
Hatvani, L., Antal, Z., Manczinger, L., Szekeres, A., Druzhinina, I.S., Kubicek, C.P., Nagy, A., Nagy, E., Vágvölgyi, Cs., Kredics, L. (2007): Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology, 97, 4: 532-537.
Kredics, L., Antal, Zs., Manczinger, L., Szekeres, A., Kevei, F., Nagy, E. (2003): Influence of environmental parameters on Trichoderma strains with biocontrol potential. Food Technology and Biotechnology, 41, 1: 37-42.
Largeteau, M. L., Rodier, A., Rousseau, T., Juarez del Carmen, S., Védie, R., Savoie, J.M. (2004): Agaricus Susceptibility to Verticillium fungicola. Proceedings of the XVIth International Congress on the Science and Cultivation of Edible and Medicinal Fungi, (Miami, USA, 14-17 March, 2004), 515-523.
Piasecka, P., Kavanagh, K., Grogan, H. (2011): Detection of sources of Lecanicilium (Verticilium) fungicola on mushroom farms. Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products. 479-484.
Rao, J.R., Nelson, D.W.A., McClean, S. (2007): The Enigma of Double-stranded RNA (dsRNA) Associated with Mushroom Virus X (MVX). Curr. Iss. Molecular Biology, 9, 2: 103-122.
Rinker, D.L., Alm, G. (2000): Management of green mould disease in Canada. Mushroom Science, 15, 2: 617-623.
Rinker, D.L., Alm, G. (2002): Evaluation of spawn and its role in green mold disease. Mushroom News, 50,7: 18-19.
Romaine, P.C., Schlagnhaufer, B. (1995): PCR analysis of the viral complex associated with La France Disease of Agaricus bisporus. Applied and Enviromental Microbiology, 61, 6: 2322-2325.
Sheepmaker, J.W.A., Geels, F.P., Smith, P.H., van Griensven, L.J.L.D. (1998): Influence of Steinernema feltiae and Diflubenzuron on Yield and Economics of the Cultivated Mushroom Agaricus bisporus in Dutch Mushroom Culture. Biocontrol Science and Technology, 8, 2: 269-275.
Szabó, Á., Molnár, A., Győrfi, J., Pénzes, B. (2009): New Data on the Mite Fauna of Hungary. Acta Phytopathologica et Entomologica Hungarica, 44, 1: 147-150.

