Terrestrial communities
Terrestrial communities
Every living organism has a spectrum of temperature (tolerance spectrum) it had adapted to. Living organisms react to changing conditions in two ways: they change their area or phenological cycle. With global warming, the area with appropriate temperature for living organisms shifted towards the poles, or higher elevations in mountainous regions. A number of studies have been dealing with the potential effects of climate change on the flora and the fauna (e.g. Thuiller et al. 2004, 2005, Kelly et al. 2008, Eaton 1996).
Kelly et al. (2008) found that in 30 years, the area of most of the 1350 mountainous plant species examined in the study shifted upwards with 64.7 m on average. Accordingly, vegetation zones shifted towards the higher regions everywhere. At the lower boundaries of these areas, plants have been unable to endure the increased temperature and the occasional weather extremes, so these species perished here and other species have taken over. When examining the reasons of this phenomenon, the researchers found that elevating snow line, increasing temperature and drought periods had decisive role in the transformation. The 99 species included in the study of Parmesan and Yohe (2003) wanders 6.2 km/decade to the North on average. 434 species changed their area according to the climate change: species of the temperate zone are expanding, but the area of the polar species is shrinking.
The problem that endangers the communities most is that living organisms do not react in the same way and extent to changing environmental conditions, therefore food networks can be transformed dramatically. Different members of the food chain react to climate change in different ways (Crick 2004, Harrington et al. 1999). For today, it has also become known that the higher the position of a species in the food chain, the more sensitive this species is to climate change (Voigt et al. 2003). It is also a fact of interest that species in close relationship do not necessarily react to the effects of climate change in the same extent (Végvári et al. 2009).
Shifted seasons:
A great number of observations support the fact that the beginning of the vegetation period had been shifted to earlier and earlier dates in the past decades (Parmesan and Yohe 2003, Visser and Both 2005). In North America, the beginning of the frost-free period has been shifted 11 days earlier during the last 60 years (Easterling et al. 2000). This results in earlier bud-breaking and animals appear earlier accordingly. The comprehensive study of Parmesan and Yohe (2003) showed that 62% of the observed 677 species start their spring life-cycle earlier than before.
According to a forecast, 75% of bird species are going to start their reproduction cycle earlier by 2080. Those bird species nesting earlier have changed their egg-laying pattern in a greater extent than those laying their eggs later in the year. Furthermore, there are species which appear earlier from their winter resorts or awaken earlier from their hibernation. At this time, not all the environmental conditions are optimal for them (e.g. American Robin (Turdus migratorius), Yellow-bellied marmot (Marmota flaviventris), etc.) (Crick 2004, Inouye et al. 2000 ).
There are some pieces of evidence that long-range migratory birds are less able to adapt to climate change compared to those bird species that migrate on shorter distances. This is especially true for those species whose migration is controlled not by the change in temperature but some other factors. Végvári et al. (2009) studied the arrival dates of 93 bird species. They found that there is a trend that these bird species arrive back earlier, but there are distinguishable groups: long-range migratory species cold adapt in a lesser, while short-range migratory species in a greater extent to the spring commencing earlier. Species changing heir quill-feathers in the summer arrive earlier than others which are moulting at their winter resort.
A good example for host-parasite relationship is the case of common cuckoo (Cuculus canorus) and its nest hosts. Möller et al. (2010) showed that long-range migratory birds are having more cuckoo eggs in their nests than short-range migratory or locally overwintering species. The reason for this lies in the different phenological response of these species. Since cuckoos are long-range migratory birds, they could not adapt to the vegetation period shifted earlier than their short-range migratory nest hosts. Therefore cuckoos arrive later, when their hosts have even already started egg-laying. This is a major disadvantage, since the cuckoos’ chances to lay their eggs in these nests is decreased, and this results in a trend showing cuckoos are laying their eggs into the nests of long-range migratory bird species.
Changes in vegetation:
As a summary, it can be stated that critical changes can be expected in most of the communities. These changes can fundamentally alter the image of a territory. This transformation process is related to the changes in the areas of the species which build up these communities. According to our models, the area of the Northern, mostly pine-leaved tree species are going to shrink in a significant extent, they can remain only in the Northern parts of their current area, or pushed back to some resorts or asylums. The proportion of deciduous trees however, is going to increase with their expansion to the North. In South, deciduous trees are going to be replaced by Mediterranean species; a mixed functionality should arise with longer vegetation period and greater amount of sequestrated carbon. Heading further to the South, the dominance is going to be taken over by bushes and grasses which will increase the expected frequency of fires (Thuiller et al. 2005).
Ecosystems at higher latitudes have been transformed in recent years as well: with the increase in temperature, the density of shrubs became greater and the Northern boundary of trees had expanded. Due to the altered albedo and energy regulation, increasing photosynthetic activity has been observed during the last 25 years. A contrasting effect is that water stress overburdens the advantage given by longer vegetation period. As a result, primary production had been increasing only until 2000 (1.4%), it is constantly decreasing since then. Due to higher air temperatures, the amount of precipitation decreased, so less water is available for the plants (Bunn et al 2007).
In a given community, replacing a species with another having the same function has a minimal effect, but if the original species becomes replaced with a species of different function, significant changes can take place. For example, if grass species will be replaced by broad-leaved deciduous tree species, the nitrogen and carbon cycle of temperate zone ecosystems will be altered in a significant extent. Besides, due to close interspecific connections, this change is going to affect other species as well (Thuiller et al. 2006.).
A consequence of the decreasing are of the Amazon forests is that evapotranspiration is going to decrease, and a warmer, dryer climate will be established. This is important because in a dryer climate, the chance of forest fires is greater. Besides, the quantity of withheld CO2 will also be decreased. Apart from anthropogenic effects, the rainforests of South America are also endangered by the changing climate. The most endangered parts of the forests are those close to the fringes of the forests. The process is accelerated by the opening of the canopy, since the more leaves are fallen, the more timber is going to be produced that is prone to be ignited by fire-causing events. This can lead to litter fires that damaged young trees, sometimes deeply penetrating into the forest and further opening the canopy cover of rainforests (Laurance and Williamson 2001). This is important because 10-15% of global CO2 emission come from the degradation of tropical forests.
Degradation of forests can decrease the amount of precipitation by 20% (Lean et Warrilow 1989; Shukla et al. 1990). Forest fires are caused by intended arson: 47000 forest fires have been observe in 1997 in the Amazon basin, the majority of which have been caused by farmers (Brown 1998). Forest fires are phenomena greatly increasing the emission of greenhouse gases (Laurance et Williamson 2001; Houghton 1991; Fearnside 2000). As we can see there are a number of feedback loops providing an aggravated outlook for the coming decades.
The terrestrial vegetation of Hungary
Hungary has a transitional position not only from climatic, but also from biogeographical aspect: the country lies on the boundary of Central European deciduous forest zone and the continental forest steppe, with significant submediterranean effects. The expected warming could affect the area of Hungary in a quite sensitive way, for a number of reasons. First of all, the boundaries of vegetation zones determined by climatic factors show increased sensitivity from the aspect of climate change. Second, due to the isolated position of the Carpathian Basin, its flora and fauna are increasedly endangered. Natural values of Hungary are already pushed back to a relatively small area and they are dramatically endangered even without considering climate change. Consequently, the impacts of climate change would certainly result in the change of natural structures, fundamental alteration in current conditions which calls the attention for the importance of adaption of nature protection and biodiversity conservation and the significance of the tasks in these fields. (Hufnagel and Sipkay 2012, Harnos et al. 2008)
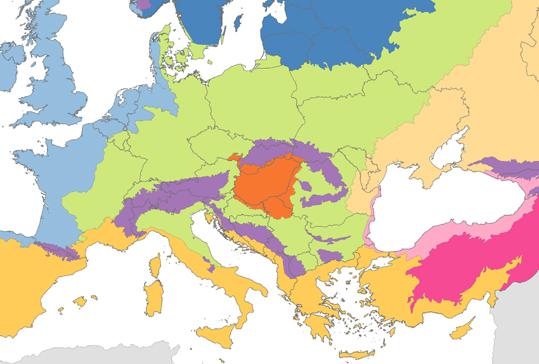
Biogeographical regions of Europe, 2005. (source: EEA)
Significant changes in the climatic conditions of Hungary, expected to be onset at the end of this century can result in the shift of biogeographical zones.
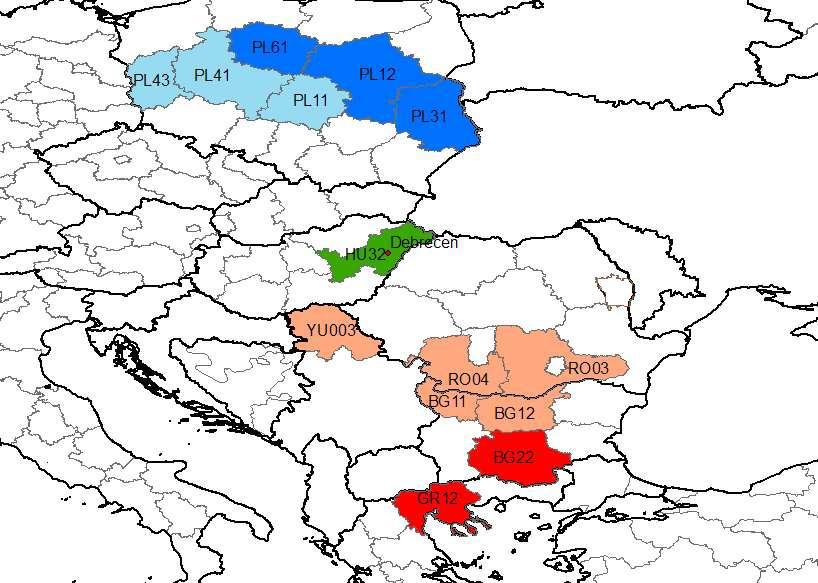
Expected shifts of climatic zones based on geographical analogy (Potential analogous NUT2 regions of Debrecen for the studied periods)
Plants adapt to the change climate on the spot, locally, if they are able to, or „wander away” using their reproductive parts. Altering their areas however, is severely hindered by the fragmentation of their current habitats due to anthropogenic activities. There is no significant connection between areas covered by natural vegetation, therefore species cannot wander, and cannot find those resorts, asylums where they can survive unfavourable periods.

Dolomite rock grass on the Odvas hill of Budaörs – home of many protected species, a victim of fragmentation (image: Gergely Petrányi)
The most endangered group of Pannon vegetation are the endemic species since their whole area is limited to the Carpathian Basin. These species include: Hungarian Iris (Iris aphylla subsp. hungarica), the iteparous perennial relic endemic Ferula sadleriana and Linum dolomiticum. Since climate change in Hungary is expected to be most significantly manifest in the drying out of wet habitats and further drying of arid habitats, increased reaction can be expected from the species favouring wet habitats (Atlantic-Submediterranean or Subatlantic species). Eurasian or cosmopolitan species with a large area are affected the least by climate change. Winners of climate change can come from drought-tolerant species with Southern area, especially if they have a good expansion ability. However, a significant part of these plants are weeds, therefore their increased expansion requires increased attention.

An endemic species of the dolomite rocks and the steppe vegetation of the warm slopes: the Linum dolomiticum (source: DINPI)
Plant species can react to climate change also by changes in phenology. According to the results of numerous studies, early spring species can react in the most spectacular way: they can shoot and blossom earlier. Long-term phenological studies have also been made in Hungary. It has been revealed that in the past 100 years the blossoming of the Black Locust shifted 3-8 days earlier in the year depending on the studied region.

Black locust (Robinia pseudoacacia) (source: http://hortiplex.gardenweb.com )
Communities of natural state and great species richness are more stable than secondary communities or those communities whose natural state has been derogated. The role of species richness in mitigating the effects of weather changes has been shown in numerous cases. Ecological systems rich in species could more effectively mitigate the negative effects of climate change. The reason why it is a significant fact is that in the densely populated Europe – and even in Hungary – there are more derogated, species-poor habitats than habitats in near-natural state.

Black Locust plantation in the Great Hungarian Plain dominated by only one tree species (source: http://zoldhullam.blog.fn.hu/ ))

Hardwood forest grove in a near-natural state ( http://www.novenyzetiterkep.hu , János Bölöni)
Animals
More than three-fourth of the currently known species are represented by invertebrate species, mostly insects and other arthropods. The role of insects is predominant not only in the biodiversity of our planet, but also fundamentally affects the functioning of near-natural or agricultural ecosystems. In this respect it is perhaps enough to think of the pollination of flowering plants, pests, agent of biological plant protection, arthropods maintaining the processes of the soil or vectors of diseases.

An inhabitant of our beech forests: the Rosalia longicorn (Rosalia alpina) (image: Gergely Petrányi)
The volatility of climate fundamentally affects the structure of insect communities, composition of regional fauna and seasonal state changes of individual, local habitats (microhabitats, biotopes). Weather exerts its direct effects through phenological and population dynamics processes which indirectly result in the transformation of areas and biodiversity.
Insect communities are not only important in themselves, but they are also indicators of the impacts of climate change. With their help we are able to gather such kinds of information which can be fathomed by no other methods.
One of the most important orders is Lepidoptera – the fourth largest insect order in Europe – consisting of 8470 species living in 35 countries. Even in Hungary, 3500 species have been indicated! Due to the richness of species, the Lepidoptera species list of a given region can give a thorough overview of the natural conditions and it is also true in reverse: butterflies and moths react sensitively to changes in abiotic environment and therefore can be used as good indicators considering climate change.

The patchlike area of the Southern Festoon (Zerynthia polyxena) follows the area of its food plant, the European Birthwort (Aristolochia clematitis) (image: Gergely Petrányi)
Well-discernible changes can be observed in the area and local abundance of wild species that can be attributed to the warming climatic trend of the 20th century. The majority of the studied Lepidoptera species showed a systematic Northern expansion during the 20th century. Depending on the species, the Northern expansion could attain 35-240 km.

Geometrids have perfectly adapted to the rocky habitats and stick to these places as well - Charissa variegata (image: Gergely Petrányi)
During the course of the 20th century, the areas of species changed systematically, and these changes were in accordance with the forecasts of global warming scenarios. Therefore, however researchers can not conduct controlled trials on the impacts of climate change on the areas of species, the repetition of similar patterns in all the studies provides a sufficiently strong evidence that climate change even currently exerts a significant impact on natural populations.

Larva of the Mountain Small White (Pieris ergane), indicator species of our untouched dolomite rock grasses (image: Gergely Petrányi)

The Death’s-head Hawk moth (Acherontia atropos) overwinters in Africa and migrates in summer to Hungary (image: Gergely Petrányi)

The Portland Moth (Actebia praecox), one of the disappearing species of our steppe fauna, last seen in the late 1970’s in Hungary (image: Gergely Petrányi)

Lignyoptera fumidaria, a steppe relict, reaches its Western boundary of its area in Hungary (image: Gergely Petrányi)
Based on the area maps of some vertebrate groups similar studies have been made by evaluating the areal situation of a species in a point (central situation, fringe situation, situation outside the area, etc.), and the proportion of species not affected by the climate change characterized by the geographical analogy, damaged, and species expected to gain benefits. (Bartholy et al 2012)
|
Taxa |
Not affected |
Loss |
New species |
|
Lepidoptera |
55-81% |
19-45% |
19-36% |
|
Mammal |
54-21% |
36-48% |
10-31% |
|
Bird |
78-23% |
15-59% |
8-18% |
|
Reptile |
51-13% |
18-21% |
28-69% |
|
Amphibian |
75-50% |
17-25% |
8-25% |
As we could see, climate change can cause significant changes in almost all communities. The main cause of the problem is the elevated concentration of greenhouse gases in the atmosphere. Due to a number of feedback loops, this causes significant changes in the climate system of the Earth with increased temperatures and more frequent climatic extremities. Every living organism tries to react to these changes, in the beginning with tolerance, then with phenological and area changes. Not all of the organisms are able to react properly, therefore systems become more vulnerable to further impacts. Demolishing communities can react back to the change of weather, further strengthening the process.
References
Asner, G. P., A. R. Townsend, B. H. Braswell: Satellite observation of El Niño effects on Amazon forest phenology and productivity, Geophys. Res. Lett., 27, 981-984, 2000.
Bartholy, J., Pongrácz, R., Nagy, J., Pieczka, I., Hufnagel, L. (2012): Regional climate change impacts on wild animals’ living territory in Central Europe – Applied Ecology and Environmental Research 10(2): 107-120.
Both, Christiaan, Sandra Bouwhuis, C. M. Lessells & Marcel E. Visser (2006): Climate change and population declines in a long-distance migratory bird - Nature 441, doi:10.1038/nature04539
Brown, P. (1998): Forest fires: setting the world ablaze - The Guardian [London], 20 March.
Bunn, A.G., S. J. Goetz, J. S. Kimball, K. Zhang (2007): Northern High-Latitude Ecosystems Respond to Climate Change - Eos, Vol. 88, No. 34
Crick Humphrey Q. P (2004): The impact of climate change on birds - Ibis (2004), 146 (Suppl.1), 48– 56
Eaton, J.G., Scheller, R.M. (1996): Effects of climate warming on fish thermal habitat in streams of the United States. – Limnol. Oceanogr. (41): 1109–1115.
Easterling, David R; Gerald A Meehl; Camille Parmesan; Stanley A Changnon;Thomas R. Karl; Linda O. Mearns (2000): Climate extremes: Observations, modeling, and impacts - Science 289, 5487
Fearnside, P. M. (2000): Global warming and tropical land-use change: greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation - Climatic Change 46:115–158.
Harnos, Zs., Gaál, M., Hufnagel, L. (szerk) (2008): Klímaváltozásról mindenkinek – Budapesti Corvinus Egyetem, Budapest. (1-197 oldal) ISBN 978-963-503-384-3
Harrington R., Stork N. E. (eds) (1995): Insects in a Changing Environment. 17th Symposium of the Royal Entomological Society. London: Academic Press.
Houghton, R. A. (1991): Tropical deforestation and atmospheric carbon dioxide - Climatic Change 19:99–118.
Hufnagel L, Sipkay Cs (szerk) (2012): A klímaváltozás hatása ökológiai folyamatokra és közösségekre – Budapesti Corvinus Egyetem, Budapest (1-530 oldal) ISBN 978-963-503-511-3
Inouye D.W., Barr, B., Armitage, K.B. & Inouye, B.D. (2000):Climate change is affecting altitudinal migrants and hibernating species - PNAS 97: 1630–1633.
Kelly, Anne E. & Michael L. Goulden (2008): Rapid shifts in plant distribution with recent climate change - PNAS vol. 105 ͉ no. 33 ͉ 11823–11826
Laurance William F. and G. Bruce Williamson (2001): Positive Feedbacks among Forest Fragmentation, Drought, and Climate Change in the Amazon - Conservation Biology, Pages 1529–1535 Volume 15
Lean, J., and D. A. Warrilow (1989): Simulation of the regional climatic impact of Amazon deforestation - Nature 342:411-413.
Lima Mauricio, Nils Chr. Stenseth and Fabian M. Jaksic (2002): Population dynamics of a South American rodent: seasonal structure interacting with climate, density dependence and predator effects - Proc. R. Soc. Lond. B 269, 2579-2586 doi: 10.1098/rspb.2002.2142
Parmesan C., Yohe G. (2003): A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421:37-43.
Sanz, J.J. 2002. Climate change and breeding parameters of great and blue tits throughout the western Palearctic. Global Change Biol. 8: 409 – 422.
Shukla, J., C. A. Nobre, and P. Sellers (1990): Amazon deforestation and climate change - Science 247:1322–1325.
Thibault, Katherine M. & James H. Brown (2007): Impact of an extreme climatic event on community assembly - PNAS vol. 105.͉no 9.
Thuiller Wilfried, Sandra Lavorel, Martin T. Sykes and Miguel B. Araújo (2005): Using niche-based modelling to assess the impact of climate change on tree functional diversity in Europe - Diversity and Distributions, (Diversity Distrib.) 12, 49–60
Thuiller Wilfried, Sandra Lavorel, Miguel B. Araujo, Martin T. Sykes, and I. Colin Prentice: Climate change threats to plant diversity in Europe - PNAS, doi10.1073pnas.0409902102
Végvári Zsolt, Veronika Bókony, Zoltán Barta and Gábor Kovács (2009) : Life history predicts advancement of avian spring migration in response to climate change - Global Change Biology, doi: 10.1111/j.1365-2486.2009.01876.x
Visser Marcel E. & Christiaan Both (2005): Proc. R. Soc. B 272, 2561–2569 doi:10.1098/rspb.2005.3356
Voigt, Winfried, Jörg Perner, Andrew J. Davis, Till Eggers. Jens Schumacher, Rudolf Bährmann, Bärbel Fabiani, Wolfgang Heinrich, Günter Köhler, Dorit Lichter,Rolf Marstaller, AND Friedrich W. Sander (2003) : Trophic levels are differentially sensitive to climate - Ecology, 84(9), pp. 2444–2453

