Aquatic and wet habitats, hydrobiological impacts
General impacts of climate change on aquatic ecosystems
Marine ecosystems are maintained by the flow of energy from primary producers at the base of food webs through to intermediate consumers, top predators (including humans), and pathogens, and then back again through decomposition and detrital pathways. Thus, marine communities are biological networks in which the success of species is linked directly or indirectly through various biological interactions (e.g., predator-prey relationships, competition, facilitation, and mutualism) to the performance of other species in the community. The aggregate effect of these interactions constitutes ecosystem function (e.g., nutrient cycling, primary and secondary productivity); through which ocean and coastal ecosystems provide the wealth of free natural benefits that society depends upon, such as fisheries and aquaculture production, water purification, shoreline protection, and recreation.
However, growing human pressures, including climate change, are having profound and diverse consequences for marine ecosystems. Rising atmospheric carbon dioxide (CO2) is one of the most critical problems because its effects are globally pervasive and irreversible on ecological timescales (Natl. Res. Counc. 2011). The primary direct consequences are increasing ocean temperatures (Bindoff et al. 2007) and acidity (Doney et al. 2009). Climbing temperatures create a host of additional changes, such as rising sea level, increased ocean stratification, decreased sea-ice extent, and altered patterns of ocean circulation, precipitation, and freshwater input.
In addition, both warming and altered ocean circulation act to reduce subsurface oxygen (O2) concentrations (Keeling et al. 2010). In recent decades, the rates of change have been rapid and may exceed the current and potential future tolerances of many organisms to adapt. Further, the rates of physical and chemical change in marine ecosystems will almost certainly accelerate over the next several decades in the absence of immediate and dramatic efforts toward climate mitigation (Natl. Res. Counc. 2011). Direct effects of changes in ocean temperature and chemistry may alter the physiological functioning, behaviour, and demographic traits (e.g., productivity) of organisms, leading to shifts in the size structure, spatial range, and seasonal abundance of populations. These shifts, in turn, lead to altered species interactions and trophic pathways as change cascades from primary producers to upper-trophic-level fish, seabirds, and marine mammals, with climate signals thereby propagating through ecosystems in both bottom-up and top-down directions. Changes in community structure and ecosystem function may result from disruptions in biological interactions. Therefore, investigating the responses of individual species to single forcing factors, although essential, provides an incomplete story and highlights the need for more comprehensive, multispecies- to ecosystem-level analyses.
The effects of rising CO2 do not act in isolation. Additional regional pressures on ocean ecosystems include intensive use of fertilizers, coastal and benthic habitat degradation, overexploitation of fish stocks, rising aquaculture production, and invasive species (Halpern et al.2008). Coastal hypoxia is increasing and expanding globally (Diaz & Rosenberg 2008). Ecosystem deterioration is intense and increasing, particularly for coastal systems, with 50% of salt marshes, 35% of mangroves, 30% of coral reefs, and 29% of seagrasses already either lost or degraded worldwide ( Jackson 2010). Thus, the integrated and synergistic effects of these multiple stressors on marine ecosystems—both CO2 and non-CO2 related—must be considered in total, not as independent issues (Doney 2010).
Ocean acidification is not a peripheral climate issue, it is the other CO2 challenge. The world's leading marine scientists are warning us that our current rates of carbon emissions are making our oceans more acidic. This is happening so fast that it poses a serious threat to biodiversity and marine life.
Left unchecked, ocean acidification could destroy all our coral reefs by as early as 2050. It also has the potential to disrupt other ocean ecosystems, fisheries, habitats, and even entire oceanic food chains.
Substantiated by the world’s leading marine scientists, these facts highlight the importance of learning more about ocean acidification and its potential impacts on our environment.
- The huge amounts of atmospheric CO2 being absorbed by the world’s oceans is making them more acidic than they have been for tens of millions of years.
· Coral Reefs provide habitat for at least a quarter of all marine species. Many of these face extinction if reefs disappear.
· The biodiversity and splendour of coral reefs are at risk of disappearing for thousands of years. This places in jeopardy an estimated 500 million people who depend on coral reefs for their daily food and income.
· The Great Barrier Reef generates over 6.5 billion dollars in tourism revenue and 63,000 jobs.
- If atmospheric CO2 can be stabilised at 450 ppm, (one possible target that has been discussed by politicians) only 8% of existing tropical and subtropical coral reefs will still be in waters of the right pH level to support their growth.
· Within decades, ocean acidification will also start to have major impacts on temperate and polar water ecosystems. In fact, colder water absorbs higher levels of CO2 than warmer water. Our polar seas are already so acidic that they are starting to dissolve some shells.
Three general rules could have been explored in the responses given by aquatic ecosystems to climate change. The first two of these rules also apply to terrestrial communities:
- Shift of areas to higher latitudes or elevations above sea level.
- Changes in phenological patterns.
- Decrease in average body weight and size both on community and population levels (Montoya and Raffaelli 2010, Daufresne et al. 2009).
Rivers, lakes, seas and oceans
Fish species often group into thermal guilds (Rahel et al. 1996, Magnuson et al. 1979) and species differ in their physiological and ecologic optimums. The increase in the temperature of the rivers might cause a number of problems in the coming years. Due to the simultaneously warming waters, fish species tend to shift to the North. Rising temperatures of Southern waters will shrink the area of species favouring warm waters, because usually their optimum range is quite narrow.
It must also be mentioned that simultaneously with the shrinking areas, the habitats of living organisms are going to shred into parts, and the remaining species become more vulnerable to the impacts of climate change (Schwartz et al. 2006). For planktons, surface water temperature is a decisive factor. In the Southern hemisphere, two phenomena have significant role in determining weather and surface water temperatures: El Niño and La Niña.
La Niña is a coupled ocean-atmosphere phenomenon that is the counterpart of El Niño as part of the broader El Niño–Southern Oscillation climate pattern. During a period of La Niña, the sea surface temperature across the equatorial Eastern Central Pacific Ocean will be lower than normal by 3–5 °C. La Niña makes the atmosphere very stable, and it is quite favourable for fish stocks.
El Niño's warm rush of nutrient-poor water heated by its eastward passage in the Equatorial Current, replaces the cold, nutrient-rich surface water of the Humboldt Current. When El Niño conditions last for many months, extensive ocean warming and the reduction in easterly trade winds limits upwelling of cold nutrient-rich deep water, and its economic impact to local fishing for an international market can be serious.
In the past decades it has been observed that the frequency of the El Niño phenomenon has grown significantly.
Coasts are experiencing the adverse consequences of hazards related to climate and sea level. Coasts are highly vulnerable to extreme events, such as storms, which impose substantial costs on coastal societies. Annually, about 120 million people are exposed to tropical cyclone hazards, which killed 250,000 people from 1980 to 2000. Through the 20th century, global rise of sea level contributed to increased coastal inundation, erosion and ecosystem losses, but with considerable local and regional variation due to other factors. Late 20th century effects of rising temperature include loss of sea ice, thawing of permafrost and associated coastal retreat, and more frequent coral bleaching and mortality.
Coasts will be exposed to increasing risks, including coastal erosion, over coming decades due to climate change and sea-level rise. Anticipated climate-related changes include: an accelerated rise in sea level of up to 0.6 m or more by 2100; a further rise in sea surface temperatures by up to 3°C; an intensification of tropical and extra-tropical cyclones; larger extreme waves and storm surges; altered precipitation/run-off; and ocean acidification. These phenomena will vary considerably at regional and local scales, but the impacts are virtually certain to be overwhelmingly negative.
Corals are vulnerable to thermal stress and have low adaptive capacity. Increases in sea surface temperature of about 1 to 3°C are projected to result in more frequent coral bleaching events and widespread mortality, unless there is thermal adaptation or acclimatisation by corals. Coastal wetland ecosystems, such as saltmarshes and mangroves, are especially threatened where they are sediment-starved or constrained on their landward margin.
Degradation of coastal ecosystems, especially wetlands and coral reefs, has serious implications for the well-being of societies dependent on the coastal ecosystems for goods and services [6.4.2, 6.5.3]. Increased flooding and the degradation of freshwater, fisheries and other resources could impact hundreds of millions of people, and socio-economic costs on coasts will escalate as a result of climate change. (IPCC Report, 2011)
Area changes of aquatic communities
Populations have three different responses when facing climate change: adaptation, area shift or extinction (Woodward et Al. 2010, Wrona et al. 2006, Perkins et al. 2010). Numerous examples can be found to these three responses. These possible responses are valid for both terrestrial and aquatic communities, and in both cases mobile and immobile populations can be distinguished. Mobile populations like birds or fish can actively change their location, while seagrasses, corals or plants can spread only passively. These immobile populations determine the structure of the community and they can tolerate changing environment for a long term, but eventually they will be unable to propagate, so their extinction is delayed (Mokany et al. 2010).
As a function of environmental factors (one of them is the climate), metapopulation dynamics and dispersal options, the spatial-temporal dispersion of individual populations generally shows nonlinear patterns. According to the climate envelope theorem, as a function of climate scenarios, based on the climate parameters of the current area and considering the physiological tolerance of a given species, the future area of species can be determined. This theorem does not include limitations arising from interspecific relations and the speed of dispersal. On a smaller spatial scale (<200 km) it seems that interspecific interactions, while on a larger scale (>200 km) climate determines the area of species (Gouhier et al. 2010). Not every species can keep up the pace of rising temperatures. Species with short lifetime and good dispersal abilities can adapt quickly to the changes, these species will form new communities with those species that disperse more slowly and having a longer life span (Montoya and Raffaelli 2010).
Due to the fragmented, isolated character of freshwater habitats, the dwellers of these habitats have limited opportunities to react to the impacts of climate change with the shift of their area (Giller et al. 2004). Supposed that a given species would require freshwater of the same temperature, a 4oC rise in temperature would necessitate a 680 km shift to the North (Woodward et al. 2010).
In marine environment sea planktons show the greatest area shifts. In large aquatic systems, dispersal is much more effective than in freshwater of terrestrial environment (Giller et al. 2004). In recent 50 years the areas of pseudo-oceanic thermophyl Ceratium (Dinophyta) species and copepod communities shifted to the North y 1100 km.
In the North Sea, during the 1970’s and 1980’s, temperate zone copepod species displaced the subarctic species. In the 1980’s, wind intensity in the region of the North Sea has increased and the water surface temperature at the Northern part of the Atlantic Ocean and in the North Sea has elevated. In 1984-85 and in 1987-88, changes of great extent could have been observed in the species composition of the planktonic communities which can be attributed to the change of several climatic parameters (wind intensity and direction, water surface temperature, NAO, Northern hemisphere temperature anomaly). The exchange of species was a positive feedback process, the transition between the two states of equilibrium has been quite rapid (Beaugrand 2004).
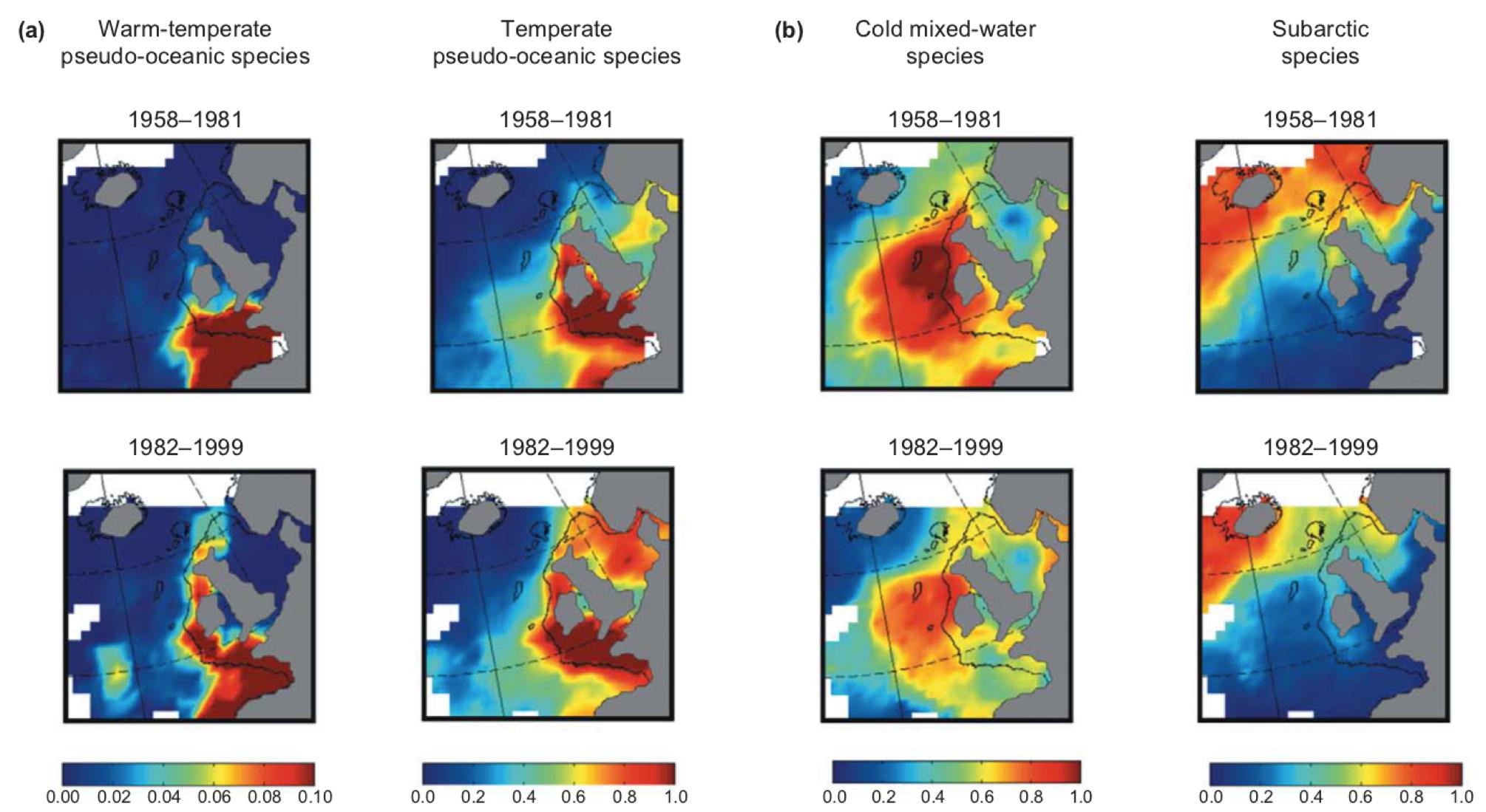
Changes taking place in the 1980’s and 1990’s in the North Sea resulting in the exchange of planktonic community (Beaugrand 2004)
The original, subarctic species are large and were present with a great biomass in the zooplankton. The size of temperate species is smaller and this species represents a much lesser biomass. Furthermore, the number of individuals of Calanus finmarchius was highest in spring, when larvae of cods were feeding on these crustaceans. The peak of C. helgolandicus replacing the C. finmarchius is in autumn, thus the main feed source of cod larvae become severely reduced or extinct (Richardson 2008).
In North-western Atlantic Ocean the same copepod communities expanded in the opposite direction: the climate in the 1980’s and 1990’s around Labrador and Newfoundland was exceptionally cold, and the Labrador stream flowing to the South became stronger. (Richardson 2008).
Changes of feeding networks in the waters of Earth
As a result of changes in the area and phenology of species, some species are going to become extinct from the feeding networks, and feeding relations become stronger or weaker. Competition of populations of the same functional groups is temperature dependent. In a rail-off experiment, above 25 oC the Cerodaphnia reticulata of smaller body size has won and the Daphnia pulex become extinct, while in lower temperatures opposing results were obtained (Lynch 1978).
Comparison of aquatic feeding networks of different latitudes show that the proportion of trophic levels and functional groups is climate dependent. Moving from North to the South, the Macroinvertebrate communities of water currents are composed of less and less species and the significance of Diptera species increases (Wrona et al. 2006).
In case of fish, higher temperatures result in increased body growth provided sufficient amount of feed is available. Lacking the sufficient amount of feed, fish inhabit colder waters or if this is not possible, hunger arises (Jansen és Hesslein 2004).
In the cold zone, warming typically causes the strengthening of higher trophic levels, increasing production and the growth of average length of food chains.
Sediment samples of the Baffin island revealed that primary production had been increased in the 20th century in the lakes (Michelutti et al. 2005). In the Loch Leven lake, Scotland, growth in the winter production of phytoplankton and the spring production of zooplankton was observed (Carvalho and Kirika 2003). Thorough analysis of lakes in the Netherlands showed that increasing primary production can be observed even at higher trophic levels. Eventually, growing production can lead to eutrophication (Mooij et al. 2005).
In the lake system of Wood River, Alaska, the earlier breaking of ice and the increase in temperature lead to a growth in the individual density of zooplankton, primarily Daphnia, and therefore the individuals of sockeye salmon (Oncorhynchus nerka) achieved higher body weight gain in their first year of life. However, this positive effect was reduced by the elevated density of older individuals (Schindler et al. 2005). According to a hydrological model, in Lake Erken, Sweden, improving light conditions can result in manifold growth of phytoplankton. Thus, light limitation will be replaced by food limitation. Greater biomass and higher proportion of diatoms due to the sedimentation of dead diatom cells leads to biomass growth of the benthic community (Blenckner et al. 2002).
In coldest habitats, the third trophic level appears only above a certain level of temperature. This process leads to strengthening top-down control and increasing production (Flanagan et al. 2003, Gyllstrom et al. 2005). Simultaneously, another effect is going to take place: in case of disturbation, there is a greater chance for species of higher trophic levels to disappear (Petchey et al. 1999, Petchey et al. 2004).
Expected impacts of climate change on freshwater communities
Freshwaters provide a disproportionately high percentage of biological diversity: they cover only 0.8% of the area of Earth, but serve as a habitat for 6% of the species. They are especially important from the aspect of ecosystem services they provide for mankind. Therefore it is of great concern that these habitats are severely endangered by climate change.
In case of freshwaters, drastic warming and more frequent drought periods can cause habitats to disappear totally. Such endangered habitats are receding glaciers, drying currents and lakes.
Climate change increases the incidence of droughts which can lead to drying out of lakes and water currents (Meyer et al. 1999). An experiment studying the monthly and three-monthly drying out of water currents found that as a consequence of drying out, the dominant green algae living on the surface of the currents had been replaced by diatoms and greater, rarer invertebrates disappeared (Woodward et al. 2010). In case of marshes and moors, longer warm and dry periods and the cessation of continuous soil water provision can accelerate filling and forestation (Burkett et al. 2005). In global scale, the amount of shallow water habitats (small water currents, rivulets, bays, marshes, moors, shallow lakes, coastal zones) may decrease (Carpenter et al. 1992).
Supposedly, inside the continents less precipitation and water flow rate can be expected. Dryer drainage basins can be more sensitive to the change of rainfall. In North America, in the Great Basin, shrinking of marshes and lakes can be expected. Seasonal distribution of precipitation can show more significant changes than annual amount of rainfall (Carpenter et al. 1992).
We also have to consider that currently applied water biological water quality systems will become useless due to increasing temperatures, since they consider an ecological state assessed in an earlier, cooler period as a reference. (Woodward et al. 2010).
Lakes react to climate change in a sensitive manner, their changes represent the impacts affected on their whole drainage basin, their state reflect the events of a longer period, therefore random, noise-like effects are excluded, but t is hard to differentiate the climatic effects from anthropogenic stressors like eutrophication, acidification and other impacts associated with land use (Adrian et al. 2009, Woodward et al. 2010). Hypolimnion of deep lakes follows every climatic change with great inertia, however, the temperature measure in 600 m depth in Lake Tanganyika increased by 0.31°C during the 20th century (OReilly et al. 2003).
Climate change has two substantial impacts on freshwater ecosystems:
- as a consequence of increasing atmospheric temperature, water temperature also going to elevate
- Change in the seasonal pattern of precipitation is going to change the hydrological conditions of aquatic systems.
Hydrological changes in freshwater systems
Lakes
In case of great lakes – just like in the case of oceans – increasing temperature advantages the layered state where upper water layers are warmer than those deeper and mixing is inhibited. The extent of mixing is also influenced by the change in wind intensity.
Continuous low water levels of greater lakes have harmful biological effects. Summer drought may cause early drying out of natural propagation places and safe living places of the offspring of fish. Water cover can disappear and several valuable habitats, like coastal stones, shallow zones inhabited by marsh vegetation etc.
Not every living organism is detrimentally affected by low water levels. Due to their mode of feeding, Anatinae favour low water levels, their number is much greater than in case of high water levels. At Lake Balaton, the Mallard is the most abundant species, but other species, like Northern Shoveler, Gadwall, Eurasian Wigeon, Northern Pintail, Garganey and Eurasian Teal can also be found. They are especially abundant in winter if the water is not frozen.

The common Mallard (Anas platyrhyncos) (image: Tibor Csörgő)

A common migratory bird and winter guest of our greater stillwaters, the Eurasian Teal (Anas crecca) (image: Tibor Csörgő)
Changes can take place even in the macrovegetation: the summer low water level is favourable for thermophyl seaweed species instead of the usual seaweed species (Potamogeton perfoliatus, Myriophyllum spicatum). Such thermophyl seaweed species is the spiny naiad (Najas marina) that additionally favours sodic, saline habitats.

Spiny naiad (Najas marina) favouring warmer, sodic water (source: http://sites-nature.aquitaine.fr )
Reed communities on the margin of the coasts have important role in the water quality of the lakes due to the filtering function of their coating of living organisms sticking to the surface of the plants. Reduction of water levels can be even positive for this vegetation. Destruction of the reeds in Lake Balaton is an increasing and accelerating process, but in years with low water levels local spreading of reeds could have been observed.


Decaying reeds in Lake Balaton (image: Csaba Sipkay)
Where the reeds form only a narrow strip along the coast of Balaton, the decrease of the water level might reach such an extent that reeds won’t have any water under them, so they can’t fulfil their filtering function.

Dried coastline at Badacsonylábdihegy at the most severe point of the 2003 drought. The original line of the coast is indicated by the stonewall on the left. (image: Csaba Sipkay)
References
Adrian, R., Deneke, R., Mischke, U., Stellmacher, R., Lederer, P. (1995): A long-term study of the Heilingensee (1975–1992). Evidence for effects of climatic change on the dynamics of eutrophied lake ecosystems. – Archiv fur Hydrobiol 133: 315–337.
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Longterm decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Babaluk, J.A., Reust, J.D., Johnson, J.D., and Johnson, L. 2000. First records of sockeye (Oncorhynchus nerka) and pink salmon (O. gorbuscha) from Banks Island and other records of Pacific salmon in Northwest Territories, Canada. Arctic 53(2):161–164.
Baltz, D.M., Moyle, P.B. (1993): Invasion resistance to introduced species by a native assemblage of California stream fishes. – Ecol Appl 3: 246–255.
Barbraud, Christophe & Henri Weimerskirch (2001): Emperor penguins and climate change Nature 411
Battarbee, Richard W. (2000): Palaeolimnological approaches to climate change, with special regard to the biological record - Quaternary Science Reviews 19 (2000) 107-124
Beaugrand, G. & Ibanez, F. 2004. Monitoring marine plankton ecosystems. II: Long-term changes in North Sea calanoid copepods in relation to hydro-climatic variability. Marine Ecology-Progress Series 284: 35-47.
Beisner, B.E., McCauley, E. & Wrona, F.J. (1996) Temperature-mediated dynamics of planktonic food chains: the effect of an invertebrate carnivore. Freshwater Biology 35, 219–232.
Beisner, B.E., McCauley, E. & Wrona, F.J. (1997) The influence of temperature and food-chain length on plankton predator–prey dynamics. Canadian Journal of Fisheries and Aquatic Sciences
Blenckner, T., Omstedt, A., Rummukainen, M. (2002): A Swedish case study of contemporary and possible future consequences of climate change on lake function. – Aquatic Sciences 64(2): 171-184.
Blenckner, T., Omstedt, A., Rummukainen, M. (2002): A Swedish case study of contemporary and possible future consequences of climate change on lake function. – Aquatic Sciences 64(2): 171-184.
Canadell, J. G., Le Quéré, C., Raupach, M. R., Field, C. B., Buitenhuis, E. T., Ciais, P., Conway, T. J., Gillett, N. P., Houghton, R. A., Marland, G. (2007): Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks, Proceedings of the National Academy of Sciences of the United States of America 104 (47): 18866-18870.
Carpenther, S.R., Fisher, S.G., Grimm, N.B., Kitchell, J.F. (1992): Global change and the freshwater ecosystems. – Annual Reviews of Ecological Systems 23: 119-139.
Carvalho, L. & Kirika, A. 2003. Changes in shallow lake functioning: response to climate change and nutrient reduction. Hydrobiologia 506 (1-3): 789-796.
Clarke, R. (1994): The pollution of lakes and reservoirs, UNEP environment library, United Nation Environmental Program, Nairobi, Kenya.
Daufresne, M., Lengfellner, K., Sommer, U. 2009. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106 (31): 12788-12793.
Dawidowicz, P., Gliwicz Z.M., Gulati, R.D. (1988): Can Daphnia prevent a blue-green algal bloom in hypertrophic lakes? A laboratory test. – Limnologica (Berlin) 19: 21–26.
Eaton, J.G., Scheller, R.M. (1996): Effects of climate warming on fish thermal habitat in streams of the United States. – Limnol. Oceanogr. (41): 1109–1115.
Ficke, A.D., Myrick, C.A., Hansen, L.J. (2007): Potential impacts of global climate change on freshwater fisheries. – Rev. Fish. Biol. Fisheries 17: 581-613.
Flanagan, K. M., E. McCauley, F. Wrona & T. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 60: 635–639.
Fritsch A.J. (1872): Die Wirbeltiere Böhmens. Ein Verzeichnis aller bisher in Böhmen beobachteten Säugetiere, Vögel, Amphibien und Fische. – Arch Naturwissensch Landesdurchforsch Böhmen 2: 1-152.
Geadke, U., Straile, D., Pahl-Wostl, C. (1998): Trophic structure and carbon flow dynamics in the pelagic community of a large lake. – In: Polis, G.A., Winemiller, K.O. (eds), Food Webs Integration of Pattern and Dynamics. Chapmann and Hall, pp. 60-71.
Giller, P. S., Hillebrand, H., Berninger, U.-G. et al. 2004. Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. -/ Oikos 104: 423-436.
Hobbie Erik A. , Stephen A. Macko Herman H. Shugart (1999): Interpretation of nitrogen isotope signatures using the NIFTE model - Oecologia (1999) 120:405-415
Hoegh-Guldberg, O., P. J. Mumby, A. J. Hooten, R. S. Steneck, P. Greenfield, E. Gomez, C. D. Harvell, P. F. Sale, A. J. Edwards, K. Caldeira, N. Knowlton, C. M. Eakin, R. Iglesias-Prieto, N. Muthiga, R. H. Bradbury, A. Dubi, M. E. Hatziolos (2007): Coral
Reefs Under Rapid Climate Change and Ocean Acidification - Science 318, 1737; doi: 10.1126/science.1152509
Hoegh-Guldberg, O. and J.F. Bruno. 2010. Impacts of climate change on the world’s marine ecosystems. Science 328:1523-1528
Holzapfel, A. M. & Vinebrooke, R. D. 2005. Environmental warming increases invasion potential of alpine lake communities by imported species. Global Change Biology 11 (11): 2009-2015.
Jansen, W., and R.H. Hesslein. 2004. Potential effects of climate warming on fish habitats in temperate zone lakes with special reference to Lake 239 of the experimental lakes area (ELA), north-western Ontario. Environ. Biol. Fishes 70: 1-22.
Jeppesen, E., Kronvang, B., Meerhoff, M., et al. (2009) Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. Journal of Environmental Quality, 48, 1930–1941.
Kiss, K.T., Ács, É., Szabó, K.É. (2007): Algák és anyagforgalmi kapcsolataik. pp. 33-49. – In: Nosek J., Oertel N. (szerk.): „A Dunának, mely múlt, jelen s jövendő…”. 50 éves az MTA Magyar Dunakutató Állomása (1957-2007). Szemelvények az Állomás tudományos eredményeiből. MTA ÖBKI – MDÁ, Göd – Vácrátót
Klapper, H. (1991): Control of eutrophication in Inland waters. – Ellis Horwood Ltd., West Sussex, UK.
Livingstone, D.M. (2003): Impact of Secular climate change ont he thermal structure of a large temperate central european lake. – Climatic Change 57: 205-225.
Lofgren, B.M. (2002): Global warming influences on water levels, ice, and chemical and biological cycles in lakes: some examples. – In: McGinn NA (ed) Fisheries in a changing climate. American Fisheries Society, Bethesda, MD, pp. 15-22.
Lynch, Michael. 1978. Complex Interactions between Natural Coexploiters--Daphnia and Ceriodaphnia. Ecology 59:552–564.
MacLeod, J.C., Pessah, E. (1973): Temperature effects on mercury accumulation,toxicity, and metabolic rate in rainbow trout (Salmo gairdneri). – J. Fisher Res Board Can 30: 485-492.
Magnuson, J. J., L. B. Crowder, and P. A. Medvick. (1979): Temperature as an ecological resource. Am. Zool. 19: 33 l-343
Magnuson, J.J. (2002.a): Future of adapting to climate change and variability. – In: McGinn NA (ed) Fisheries in a changing climate. American Fisheries Society, Bethesda, MD, pp. 283-287.
Matthews, W.J., Marsh-Matthews, E. (2003): Effects of drought on fish across axes of space, time and ecological complexity. – Freshwater Biol 48: 1232-1253.
McDonald, J.H., B.C. Verrelli and L.B. Geyer. 1996. Lack of geographic variation in anonymous nuclear polymorphisms in the American oyster, Crassostrea virginica. Molecular Biology and Evolution 13: 1114-1118.
Meerhoff, M., Fosalba, C., Bruzzone, C., Mazzeo, N., Noordoven, W. & Jeppesen, E. (2006): An experimental study of habitat choice by Daphnia: plants signal danger more than refuge in subtropical lakes. - Freshwater Biology 51: 1320-1330.
Michelutti N, Wolfe AP, Vinebrooke RD, Rivard B, Briner J (2005) Recent primary production increases in arctic lakes. Geophys Res Lett 32:L19715.
Mokany K, Richardson AJ, Poloczanska ES, Ferrier S, The CSIRO CAF Biodiversity Working Group. 2010. Uniting marine and terrestrial modelling of biodiversity under climate change. Trends in Ecology and Evolution. 25: 550-551.
Montoya JM, Raffaelli D. (2010): Climate change, biotic interactions and ecosystem services - Philos Trans R Soc Lond B Biol Sci. 2010 Jul 12;365(1549):2013-8.
Mooij, W. M., Domis, L. N. D., Janse, J. H. 2009. Linking species- and ecosystem-level impacts of climate change in lakes with a complex and a minimal model. Ecological Modelling 220 (21): 3011-3020.
Moss B, McKee D, Atkinson D, Collings SE, Eaton JW, Gill AB, Hatton HK, Heyes T and Wilson D (2003) How important is climate? Effects of waming, nutrient addition and fish on phytoplankton in shallow lake microcosms. J Appl Ecol 40: 782-792
Murty, A.S. (1986.a): Toxicity of pesticides to Fish. I. - II. CRC Press, Boca Raton, FL.
Nussey, G., van Vuren, J.H.J., du Preez, H.H. (1996): Acute toxicity of copper on juvenile Mozambique tilapia, Oreochromis mossambicus (Cichlidae), at different temperatures. – S Afr J Wildlife Res 26: 47-55.
O’Reilly, C.M., Alin, S.R., Plisnier, P.D., Cohen, A.S., McKee, B.A. (2003): Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. – Nature 424: 766-768
Palmer, M. D. , S. A. Good, K. Haines, N. A. Rayner, P. A. Stott, (2009): A new perspective on warming of the global oceans GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L20709, 5 PP., 2009 doi:10.1029/2009GL039491
Perkins DM, Mckie BG, Malmqvist B et al. (2010) . Environmental Warming and Biodiversity-Ecosystem Functioning in Freshwater Microcosms: Partitioning the Effects of Species Identity, Richness and Metabolism. ADV ECOL RES vol. 43, 177-209.
Petchey O. L., McPhearson P. T., Casey T. M., Morin P. J. 1999 Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72. (doi:10.1038/47023)
Petchey, O.L., Hector, A. & Gaston, K.J. (2004). How do different measures of functional diversity perform? Ecology, 85, 847–857.
Poff, N.L., Brinson, M.M., Day, J.W. (2002): Aquatic ecosystem & Climate change. Potential impacts on inland freshwater and coastal wetland ecosystems in the United States. – Pew Center on Global Climate Change. pp. 44.
Quinlan, R., Douglas, M.S.V., Smol, J.P. (2005): Food web changes in arctic ecosystems related to climate warming. – Global Change Biology 11(8): 1381-1386.
Rahel Frank J., Christopher J. Keleher, and Jefry L. Anderson (1996): Potential habitat loss and population fragmentation for cold water fish in the North Platte River drainage of the Rocky Mountains: Response to climate warming - Limnol. Oceanogr., 41(5), 1996, 1116-l 123.
Reynolds, C.S. (1997): Vegetation processes in the pelagic: A model for ecosystem theory. – Ecology Institute.
Richardson, A. J. 2008. In hot water: zooplankton and climate change. ICES Journal of Marine Science 65 (3): 279-295.
Rolinski, S., Horn, H., Petzoldt, T., Paul, L. (2007): Initifying cardinal dates in phytoplankton time series to enable the analysis of long-term trends. – Oecologia 153: 997-1008.
Schindler, D. E., D. E. Rogers, M. D. Scheuerell, and C. A. Abrey. 2005. Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology 86:198- 209.
Schwartz Mark W, Louis R. Iverson, Anantha M. Prasad, Stephen N. Matthews & Raymond J. O’Connor et al. (2006): Predicting extinctions as a result of climate change - Ecology, 87(7), 2006, pp. 1611–1615
Sipkay Cs,.Kiss, K. T., Vadadi-Fülöp, Cs., Hufnagel, L. (2009b): Trends in research on the possible effects of climate change concerning aquatic ecosystems with special emphasis on the modelling approach. - Applied Ecology and Environmental Research 7(2): 171-198.
Sipkay, Cs Kiss K.T, Vadadi-Fülöp Cs, Homoródi R and L. Hufnagel (2012): Simulation modeling of phytoplankton dynamics in a large eutrophic river, Hungary — Danubian Phytoplankton Growth Model (DPGM) - Biologia(Bratislava) Section Botany, Volume 67, Number 2, 323-337, DOI: 10.2478/s11756-012-0004-2 (IF 2010: 0,609)
Sipkay Cs, Kiss, K. T., Vadadi-Fülöp, Cs., Hufnagel, L. (2009): Trends in research on the possible effects of climate change concerning aquatic ecosystems with special emphasis on the modelling approach. - Applied Ecology and Environmental Research 7(2): 171-198.
Sipkay, Cs., Horváth, L., Nosek, J., Oertel, N., Vadadi-Fülöp, Cs., Farkas, E., Drégelyi-Kiss, Á., Hufnagel, L. (2008): Analysis of climate change scenarios based on modelling of the seasonal dynamics of a Danubian copepod species - Applied Ecology and Environmental Research 6(4): 101-108
Straile, D. (2005): Food webs in lakes – seasonal dynamics and the impacts of climate variability. – In: Belgrano, A., Scharler, U.M., Dunne, J. & Ulanowicz, R.E. Straile, D., Livingstone, D.M., Weyhenmeyer, G.A., George, D.G. (2003): Complex effects of winter warming ont he physico-chemical characteristic of a deep lake.
Thackeray, S.J., Jones, I.D., Maberly, S.C. (2008): Long-term change in the phenology of spring phytoplankton: species-specific responses to nutrient enrichment and climatic change. – Journal of Ecology 96: 523-535.
Vadadi-Fülöp Cs., D. Türei, Cs. Sipkay, Cs. Verasztó, Á. Drégelyi-Kiss, L. Hufnagel (2009): Comparative Assessment of Climate Change Scenarios Based on Aquatic Food Web Modeling - Environmental Modeling and Assessment 14(5) : 563-576
Vadadi-Fülöp, Cs., Hufnagel, L., Sipkay, Cs., Verasztó, Cs. (2008): Evaluation of climate change scenarios based on aquatic food web modelling - Applied Ecology and Environmental Research 6(1):1-28.
Vadadi-Fülöp, Cs., Cs Sipkay , G. Meszaros , L. Hufnagel (2012): Climate change and freshwater zooplankton: what does it boil down to? - AQUATIC ECOLOGY 46(4): 501-519 DOI: 10.1007/ s10452-012-9418-8 Published: DEC 2012 (IF2011: 1,365)
Van Doorslaer W, Robby Stoks, Ine Swillen, Heidrun Feuchtmayr, David Atkinson, Brian Moss, Luc De Meester (2010): Experimental thermal microevolution in community-embedded Daphnia populations –Climate Research Vol. 43: 81–89, 2010 doi: 10.3354/cr00894
Van Doorslaer W, Stoks R, Duvivier C, Bednarska A, De Meester L (2009) Population dynamics determine genetic adaptation to temperature in Daphnia. Evolution 63: 1867–1878
Weyhenmeyer, G.A., Blenckner, T., Pettersson, K. (1999): Changes on the plankton spring outburst related to the North Atlantic Oscillation. – Limnology Oceanography 44: 1788-1792.
Woodward G, Perkins DM, Brown LE (2010) . Climate change and freshwater ecosystems: impacts across multiple levels of organization. PHILOS T R SOC B vol. 365, (1549) 2093-2106.
Wrona, F.J., T.D. Prowse, J.D. Reist, J.E. Hobbie, L.M.J. L´ evesque. & W.F. Vincent. 2006. Climate change effects on aquatic biota, ecosystem structure and function. - Ambio 35: 359–369.

